6s Orbital Diagram
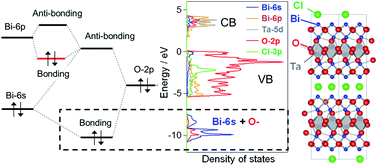
Strong Hybridization Between Bi 6s And O 2p Orbitals In Sillen Aurivillius Perovskite Bi4mo8x M Nb Ta X Cl Br Visible Light Photocatalysts Enabling Stable Water Oxidation Journal Of Materials Chemistry A

Atomic Orbital Wikipedia

8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts

Molecular Orbitals Diagram Left And Isodensity Representations Download Scientific Diagram

Orbital Filling Diagrams The Cavalcade O Chemistry

Strong Hybridization Between Bi 6s And O 2p Orbitals In Sillen Aurivillius Perovskite Bi4mo8x M Nb Ta X Cl Br Visible Light Photocatalysts Enabling Stable Water Oxidation Journal Of Materials Chemistry A
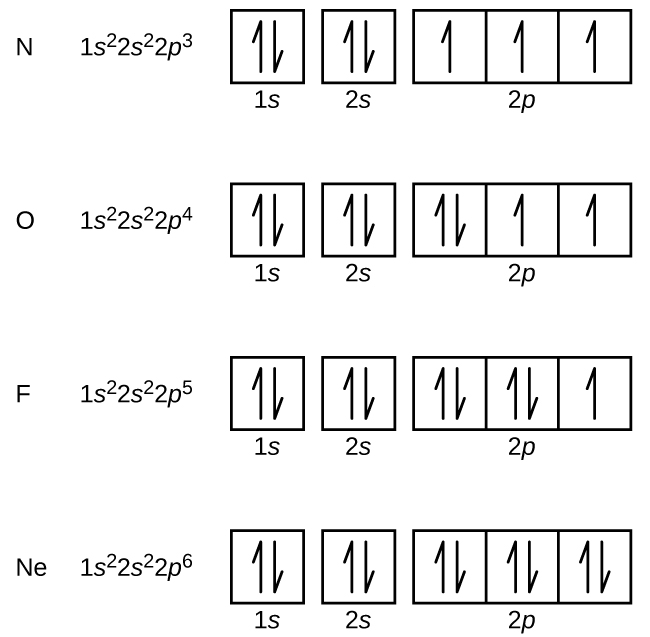
For example, an s subshell is represented by 1 box, and a p subshell is represented by 3 boxes.
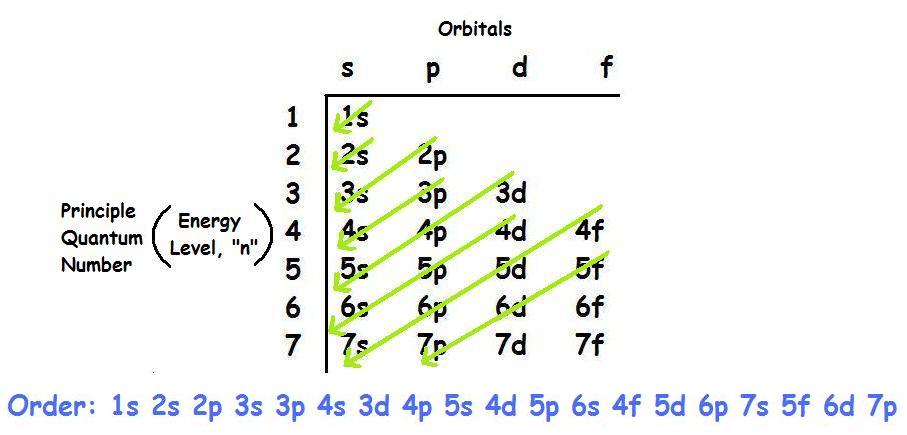
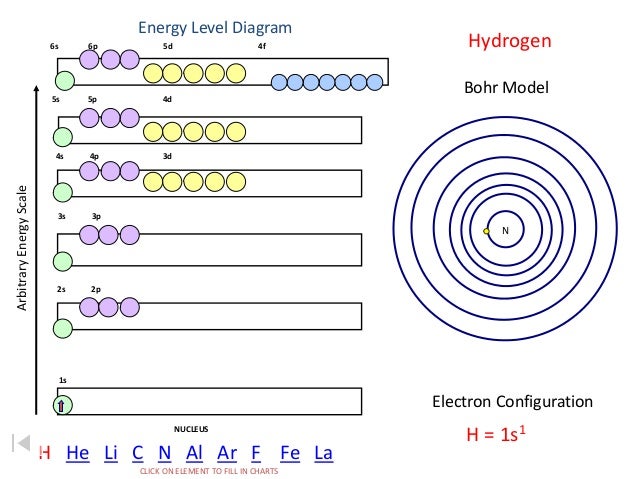
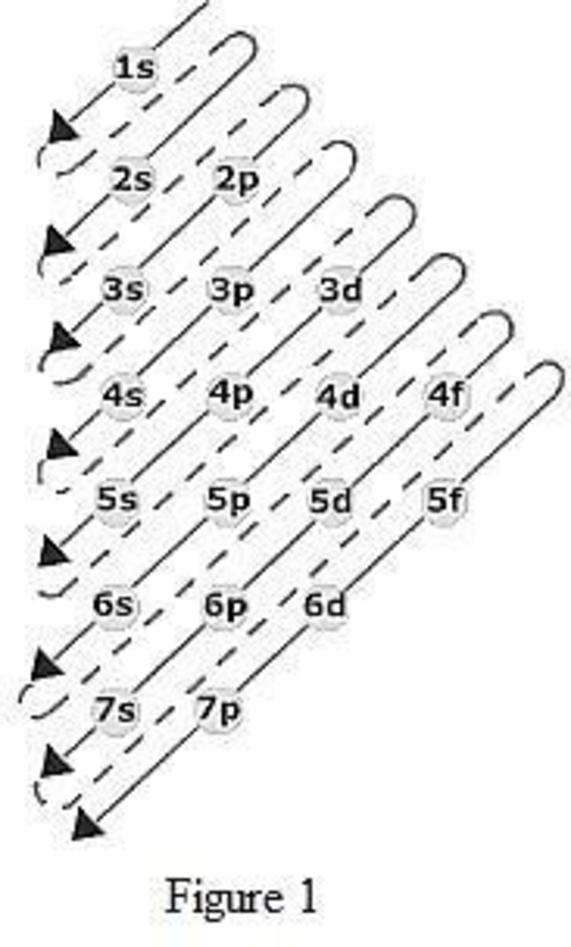
6s orbital diagram. Sample electron order filling for calcium. The left diagram shows the deviated order, 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy.
The key aspects of bonding and reactivity in plumbylenes are dictated by the inert pair effect, whereby the combination of a widening s–p orbital energy gap as a trend down the group 14 elements and a strong relativistic contraction of the 6s orbital lead to a limited degree of sp hybridization and the 6s orbital being deep in energy and inert. Layer (row #), s = orbital type, power of 2 = the 2 electrons in the 1s. MO diagram of Au13Cl2-X (X = P, C).Values in brackets show the orbital contributions of 5d(Au), 6s(Au), 6p(Au) and ligands.
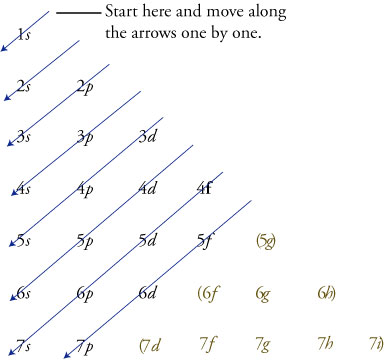
(click to see image?. Follow the arrows from the top 1s 2s 2p 3s 3p. Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.
Choose any element #1-. Noble gas notation:Kr5s24d105p4 2. Now for the pain of doing the orbital filling diagram of lead:.
Which orbital is higher in energy - 4f or 6s?. π-bonding perpendicular to the plane. MO Diagram for SF6 S 3p S 3s SF6 MOs bonding MOs antibonding MOs non-bonding S AOs F LCAOs a1g t1u F 2p a1g t1u eg t1g t2g t2u a1g eg t1u 2a1g 3a1g 2t1u 4t1u 14 × 6 × t1u The resulting MO diagram contains:.
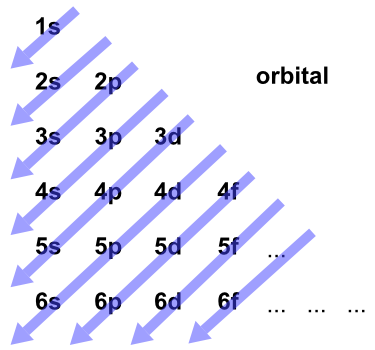
You have to use this diagram to know the order of the orbitals. FIGURE 1.6 The Aufbau principle is illustrated in the diagram by following each red arrow in order from top to bottom:. So, the most frequently used names for the s orbitals are 1s, 2s, 3s, 4s, 5s, 6s and 7s.
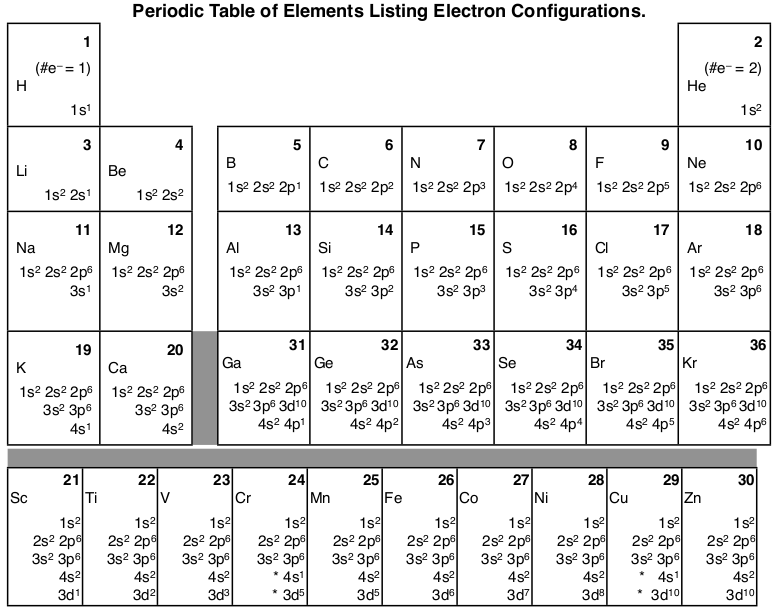
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 1:. Note that the 4f sublevel does not fill until just after the 6s sublevel. *Isoelectronic – atoms or ions of different elements that have the same electronic configuration.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. Go diagnol so firsts comes 1s. After filling 6s, electrons would fill.
5s 5p 5d 5f. Xe 6s2 4f14 5d4 g) Tellurium Orbital notation:21s 2s2 2p6 3s 3p 4s2 3d10 4p6 5s2 4d10 5p4 Orbital notation + Arrows:. 2h9 1979 Yamaha Xs1100 Wiring Diagram European;.
Orbital adalah wilayah atau daerah dalam ruang di sekitar inti atom yang memiliki kemungkinan tertinggi untuk bisa menemukan elektron. Xe6s 2 4f 14 5d 6 or Xe4f 14 5d 6 6s 2. Intramolecular Diels-Alder – 1,3,9-decatrien-8-one;.
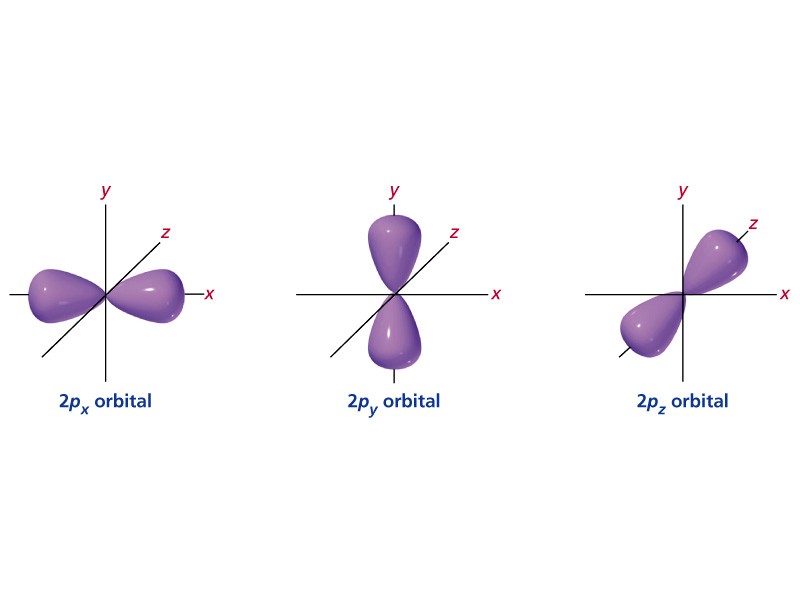
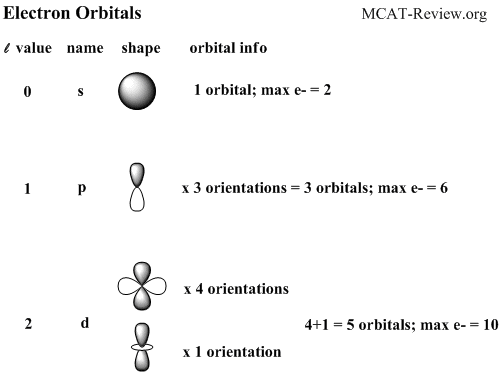
Of the four, we'll be concerned primarily with s and p orbitals because these are the most common in organic chemistry. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. The Woodward Hoffman description of the Diels-Alder;.
The total number of electrons in a given atom is equal to its atomic number. 4s 4p 4d 4f. Write the ground state electron configuration for neutral atom Iodine.
Aulon You May Use A Noble Gas Core. Write the electron configuration. There are 78 inner electrons and 4 valence electrons.
Since we know that energy of an electron is determined by its principal quantum number ‘n’ denotes the principal energy level of an electron. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. According to quantum theory, the principal quantum number n specifies the size and energy level of the.
Point Group = D 4h. The oddity is the position of the 3d orbitals, which are shown at a slightly higher level than the 4s. Then go diagonal again then comes 2p and 3s.
Please Make Sure To Get The Energy Order Correct For The Sd And 6s Sublevels (which Is Higher Energy?) Please Submit Your Work As A PDF. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. In other words, when we.
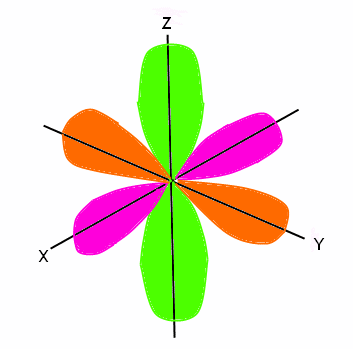
Shapes of Orbitals and Electron Density Patterns. Cara menentukan konfigurasi elektron spdf ke dalam orbital – orbital dikenal dengan prinsip Aufbau. The orbital letters you need to remember are s, p, d and f.
How many unshared pairs of electrons are in this orbital diagram?. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 6. This means that the s,p,d,f electron configuration for Barium must end with 6s^2.
What must be true for two electrons in one orbital?. The vectors lie along a C 2 rotation axis, there the p orbitals can be separated into one set of four perpendicular to the plane and one set of four parallel to the plane. The 1s orbital is the smallest, and the 7s orbital is the largest.
View Available Hint(s) 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 58 50 50 51 6s 6p 6d 75 7 7 Submit Previous Answers. What is the electron configuration B (Boron). 1s 2 2s2 2p6 3s2 ↑↑↑ 1 = 1.
Draw The Orbital Energy Diagram (hint With A Box For Each Orbital And An Up Or Down Arrow For Each Electron) For The Following Species:. Element #2 0 Element Symbol:. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 4 6s 2:.
3s 3p 3p. - Part Use The Orbital-filling Diagram To Show The Electron Configuration Of Gallium, Ga. When electrons occupy orbitals of equal energy, one electron enters each orbital until all the orbitals contain one electron with the same spin direction.
One orbital - 6s which can hold 2 electrons. Which atomic orbital is of higher energy, a 4f or a 5p orbital?. Orbitals Chemistry (s, p, d, and f Orbital) - Atomic Orbitals are of four different kinds, denoted s, p, d, and f, each with a different shape.
This suggests that the 6s orbital, which has most electron density farther out from the nucleus, has electron(s) that are less attracted to the nucleus (smaller Z_(eff)) than those in either the 5d or 4f orbitals, and thus contains the first electron that is easiest to remove during ionization. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. Click Within An Orbital To Add Electrons.
The quantum numbers n = 6 and l = 0 specify a 6s orbital. When drawing orbital diagrams, each orbital is represented as a box. 1s, 2s, 2p, 3s, etc.
The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. Each orbital can hold a maximum of _____ electrons. Pada penyusunan diagram orbital, sebuah elektron disimbolkan dengan anak panah menghadap ke atas yang melambangkan elektron dengan spin +½, atau menghadap ke bawah yang melambangkan elektron dengan spin -½.
Ca 6s 5s 4s 3s 2s 6p 5d 4d 3d 4f 5p 4p 3p 2p 1s 1s22s22p63s23p64s2 Figure 3. Use The Buttons At The Top Of The Tool To Add Sublevels. To determine the orbital from a given pair which has higher energy in a many electron atom.
We will use the short form, but show the actual orbitals, where each orbital picture is represented by a horizontal line. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2 The rules are the same, so I’ll just bite the metaphorical bullet and write it out here:.
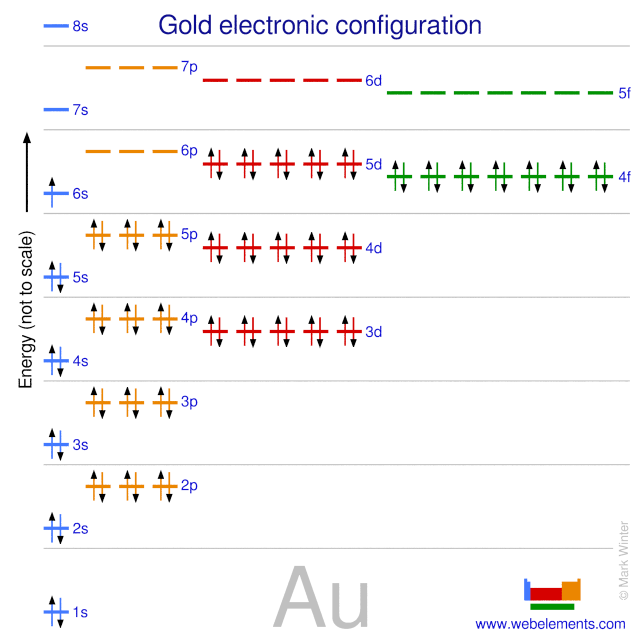
Determine if the ion is diamagnetic or paramagnetic. The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. These letters represent the angular momentum quantum number l, but all you need to remember is that the first energy level only has an s orbital, the second energy level has s and p, the third energy level has s, p and d, and the fourth energy level has s, p, d and f.Any higher energy levels have additional shells, but these just.
Then do diagonal again comes 2s. The electron in the 6s orbital of Bi 3+ on the other hand extends to the periphery of the cation resulting in an overlap and hybridization with the anion orbitals. Electron Configurations and Electron Orbital Diagrams Electron Configurations Ex.
Orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10. How many unshared pairs of electrons are in this orbital diagram. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2:.
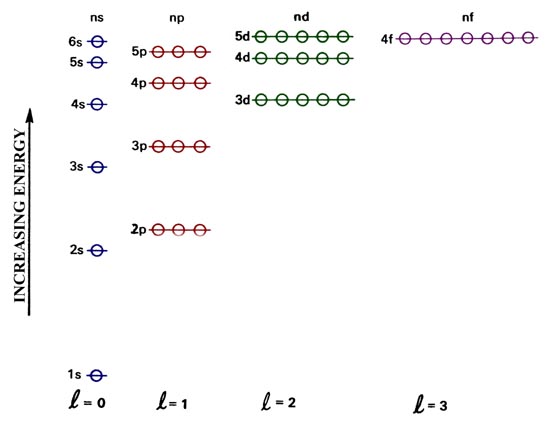
Check your answer here. The diagram (not to scale) summarizes the energies of the orbitals up to the 4p level. 2 i 6S 4 8S 6 3 σ h 6σ d A 1g 11 111 111.
Menurut prinsip Aufbau, elektron dalam atom harus memiliki energi terendah, artinya elektron harus terlebih dahulu menghuni orbital dengan energi terendah, lihat diagram tingkat energi orbital berikut:. • 6 non-bonding MOs derived from F 2s AOs • 4 bonding and 4 anti-bonding MOs of a. For the orbital diagrams:.
If the principle energy level is n=1 then the type of sublevel is 1sN=2---> type of sublevel is 2s and 2pN=3---> type of sublevel is 3s, 3p, and 3dN=4. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. There are 118 elements in the periodic table.
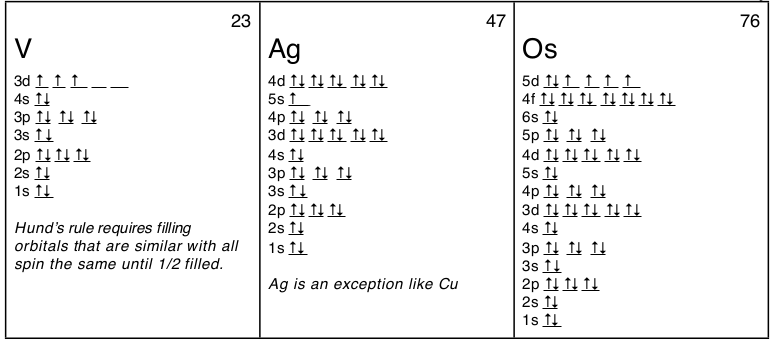
2,3-Dimethylbutadiene and Acrolein(propenal) Quinone as Dienophile – Steroid Framework. For Ag the abbreviation would be:. Kr5s 1 4d 10 (see orbital diagram above), and for Os:.
The electron in the 4f-orbital of Ce 3+ is relatively close (on average about –25 pm) to the nucleus and it is screened from the chemical environment by the 5s and 5p electrons. The representation on the right is called an orbital diagram. The 6th row, s.
Learn more about atomic orbital at Byjus. Look at the aufbau diagram, Figure 5.7 on page 133. Answer to Write orbital diagram for Au+.
Recall that d block elements use ns, np and (n-1)d orbitals for bonding, and that (n-1)d orbitals are lower in energy than ns and np orbitals. Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. It may be simpler to think of these two letters in terms of orbital shapes (d and f aren't described as readily).However, if you look at a cross-section of an orbital, it isn't uniform.
Condensed configuration partial orbital diagram full configuration 5s 1 4d 5 condensed configuration partial orbital diagram full configuration 6s 2 6p 2 5p 12. This means that the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals. Also, the s orbitals occur singly.
An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. 1s222s262p6 23s 103p 4s 3d10 4p6 5s 4d 5p6 6s2 4f14 5d4 Orbital notation + Arrows:. The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z).
Orbital explanation for the endo rule;. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. What is a orbital diagram and what is the diagram for neon?.
Each orbital is spherical, with the nucleus at the center of the sphere. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 3 ,. You will need to be able to construct orbital diagrams the go up to the 3p sub-level.
Just remember that the abbreviations require that you use oble gases only and that you use a noble gas with fewer electrons. Ok starting from 1s you have to go diagonally. Electron Configuration Chart for All Elements in the Periodic Table.
Orbital diagram and electron configurations. Which element, when it loses one electron, will have the electron configuration. Orbital Chart Overhead continued 2 21 Flinn cientific nc ll ights eserved A sample electron order filling diagram is shown in Figure 3 for calcium.
6s Orbital Diagram for Elements with Xenon as a Base Name and Date Element Name. Working out which product is endo;. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 1 5d 1 6s 2:.
7/8/ PM.Xenon, electron configurationWhat is the orbital notation of xenon. MO diagram of Au13Cl3-X (X = P, C).Values in brackets show the orbital. And it is in period 6, so the 6s orbital will be the orbital that holds its valence electrons.
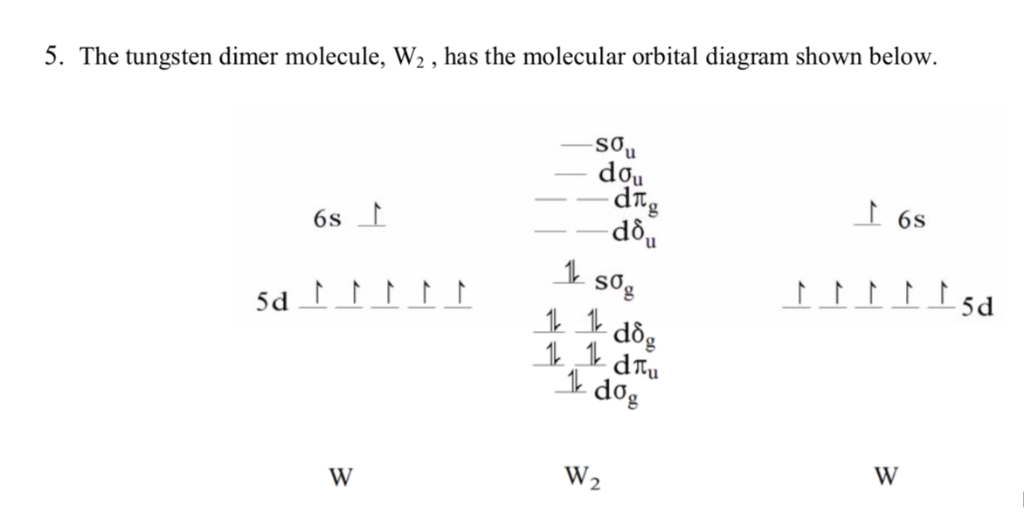
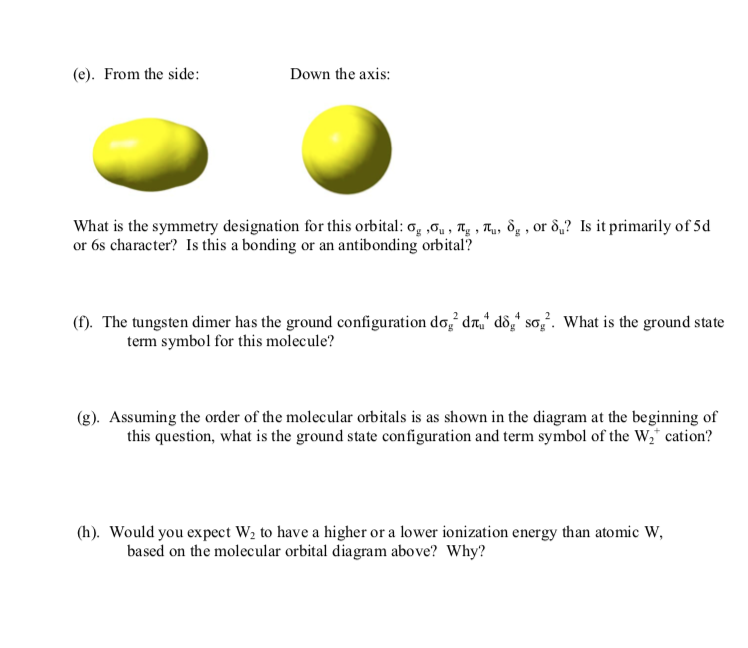
5 The Tungsten Dimer Molecule W2 Has The Molecu Chegg Com

Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Http Pnhs Psd2 Org Documents Lcasey Pdf

Figure 2 From Covalent Gold Semantic Scholar
1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts
Q Tbn 3aand9gcslroe9l0dw1qgiam3sxxwyzd0d8xvfoepo0hmycba Usqp Cau
1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts

Chapter 4 Electrons In Atoms Ppt Video Online Download
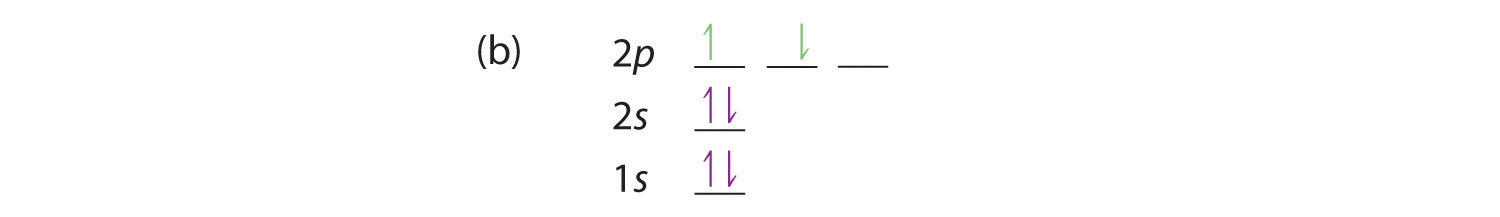
Building Up The Periodic Table

Upper Radial Component Of The Cs 6s Orbitals Thick Solid Line Download Scientific Diagram
Electron Configuration Wyzant Resources
Question Socratic

Electron Configuration Wikipedia
1

Orbital Diagrams And Electron Configurations Vocabulary 1 Electron Configuration 2 Aufbau Principle 3 Pauli Exclusion Principle 4 Electron Spin 5 Hund S Ppt Download

C The Electron Configuration Of The Noble Gases Core Notation
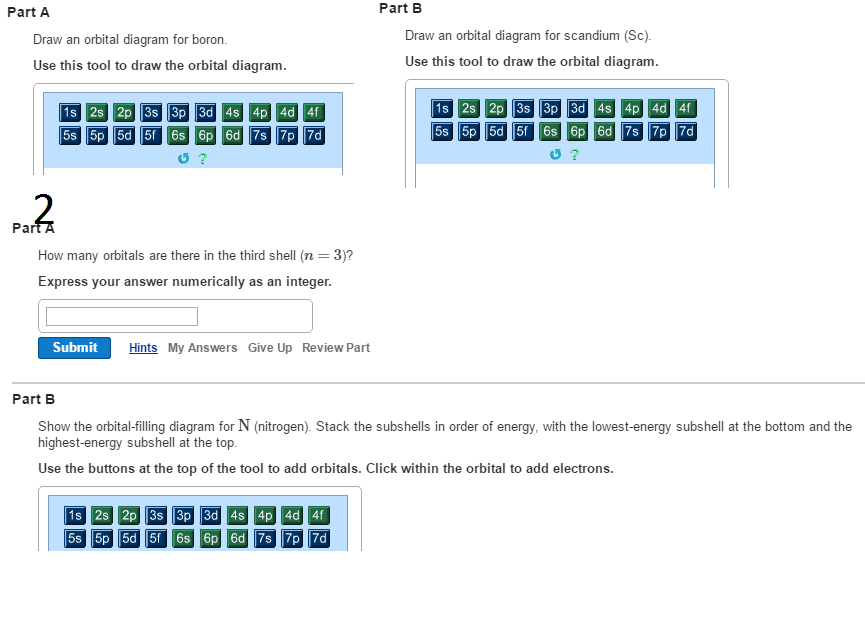
Solved Draw An Orbital Diagram For Boron Use This Tool T Chegg Com

Clarifying Electron Configurations Chemical Education Xchange
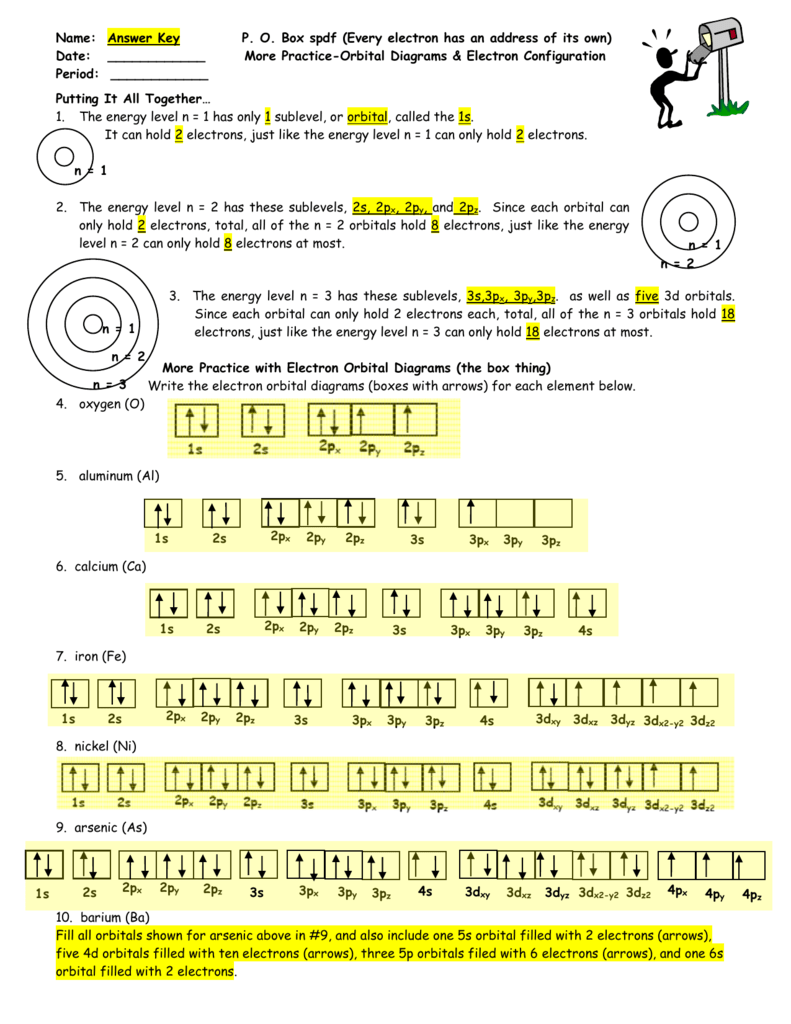
Po Box Spdf Worksheet Answer Key

The Relativistic Contraction Of The 6s Orbital And Expansion Of The 5d Download Scientific Diagram

Dublin Schools Lesson Orbital Diagrams And Electron Configurations

A Molecular Orbital Plots Of Ir 5d Orbitals In R 3 Irf 8 B Download Scientific Diagram
Q Tbn 3aand9gcseyobbyyxbjn6tvzukgg8pqxexr4l9yuq G9cwemw2k9xszngu Usqp Cau

Illustration Of The Orbital Interactions That Lead To Lone Pair Download Scientific Diagram

Atomic Orbital Filling Order Ppt Video Online Download
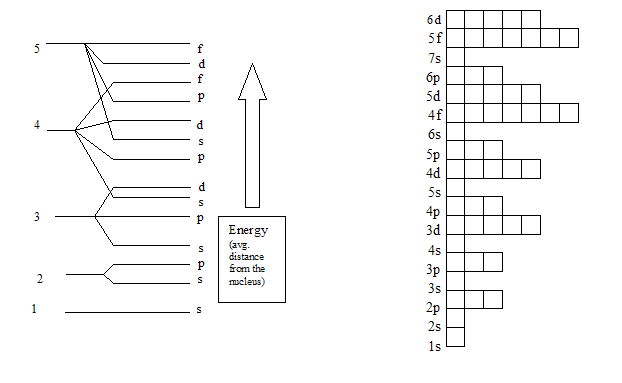
Summary Of Electron Configurations

Energy Level Diagram Arbitrary Energy Scale 1s 2s 2p 3s 3p 4s 4p 3d 5s 5p 4d 6s 6p 5d 4f Nucleus Bohr Model Electron Configuration Click On Element To Ppt

Chemistry The Central Science Chapter 6 Section 8

Molecular Orbital Diagrams For Vcu X Vag X And Vau X X Download Scientific Diagram
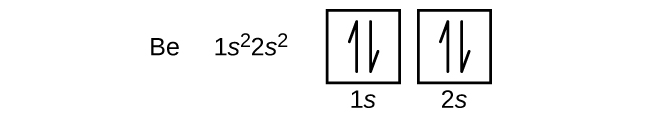
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
What Is An Abbreviated Orbital Diagram Quora
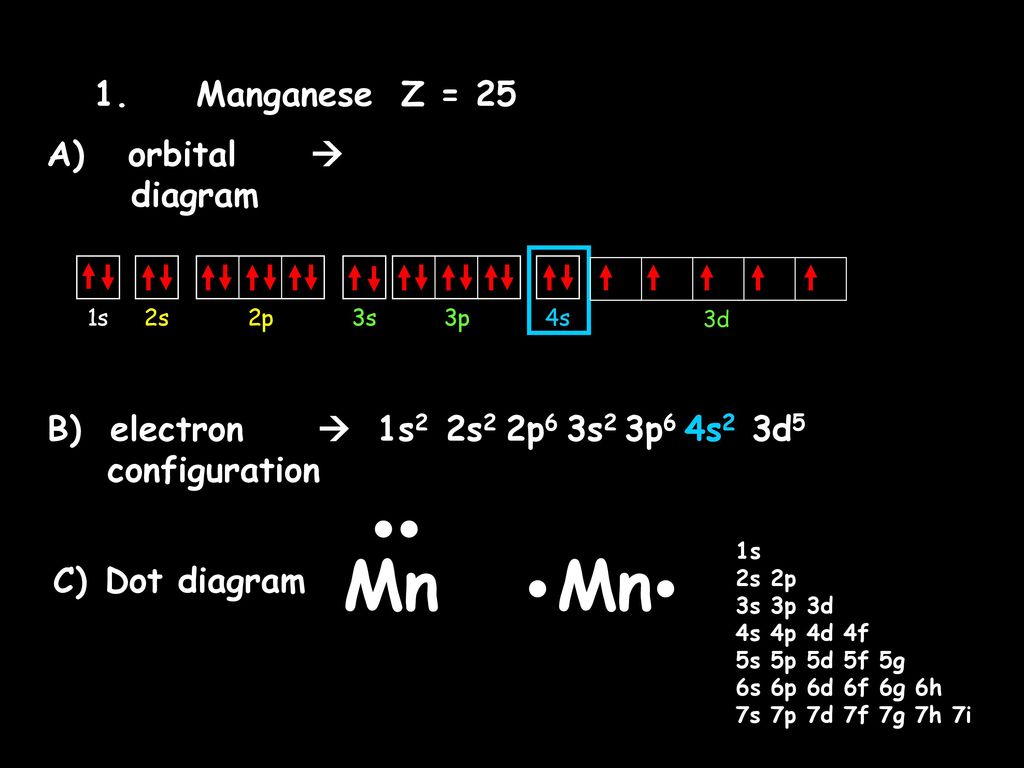
Orbital Diagrams Electron Configurations And Dot Diagrams Ppt Download
Electron Configurations And Atomic Orbital Diagrams Chemistry
5 The Tungsten Dimer Molecule W2 Has The Molecu Chegg Com

Dublin Schools Lesson Orbital Diagrams And Electron Configurations
What Are The Four Quantum Numbers Example
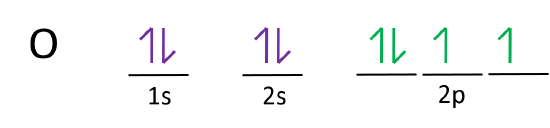
2 2 Electron Configurations Chemistry Libretexts

Radial Distribution Of The 6s And 7s All Electron And Download Scientific Diagram
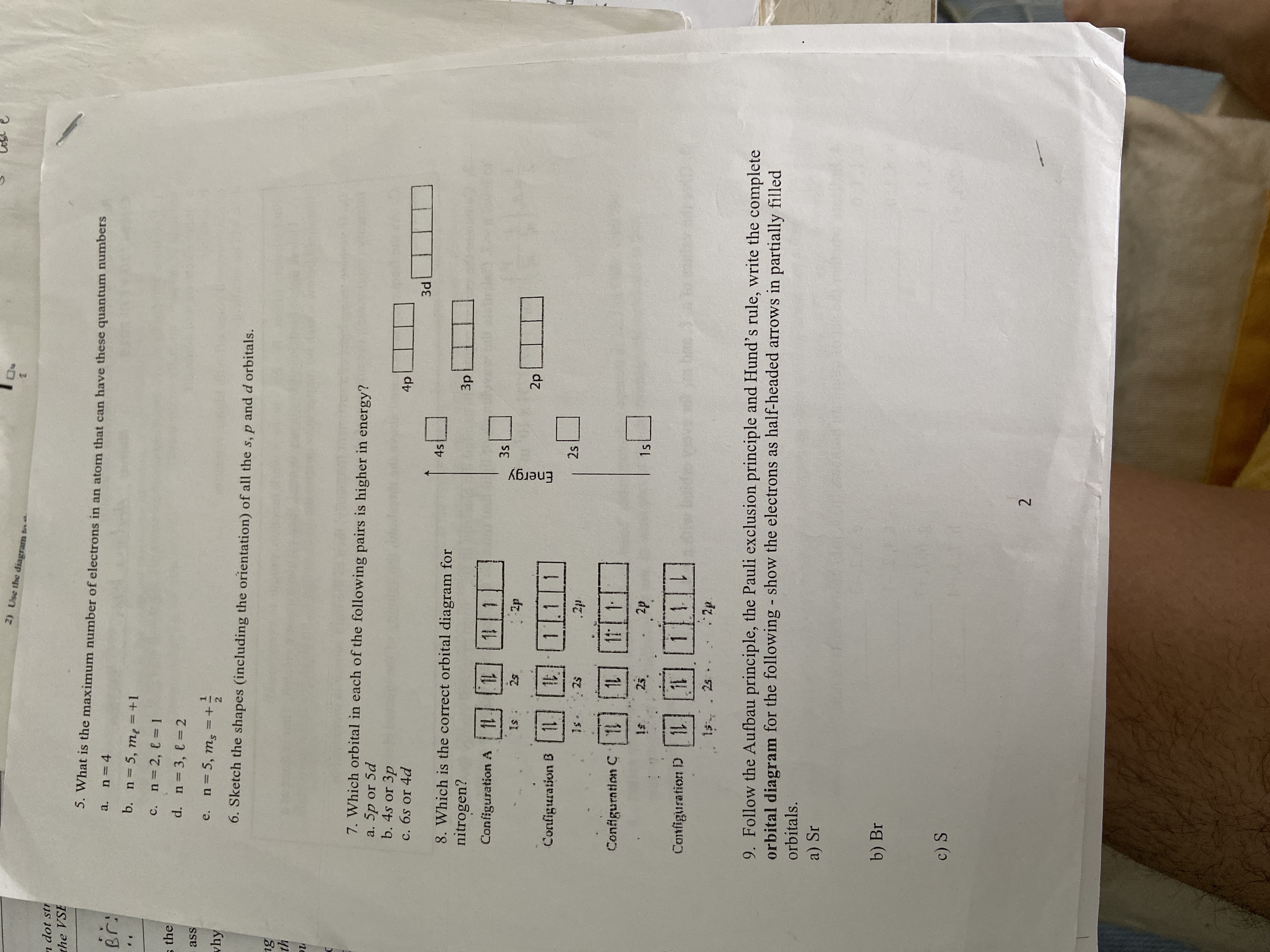
Answered 2 Use The Diagram N Dot Str The Vse 5 Bartleby
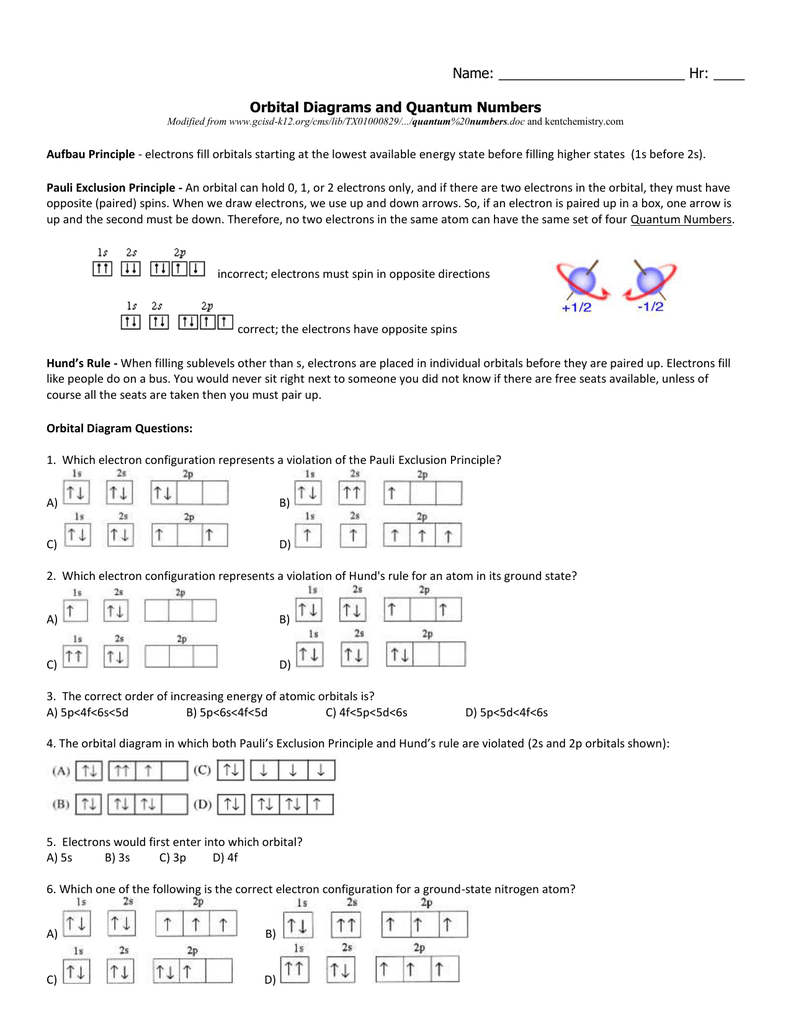
Worksheet Quantum Numbers

Quantum Numbers
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry

Electronic Structure Of Atoms Electron Configurations Chemistry
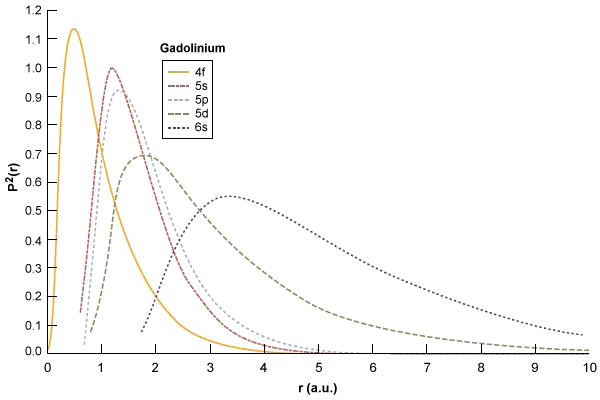
The Lanthanides

Electron Configuration Boundless Chemistry
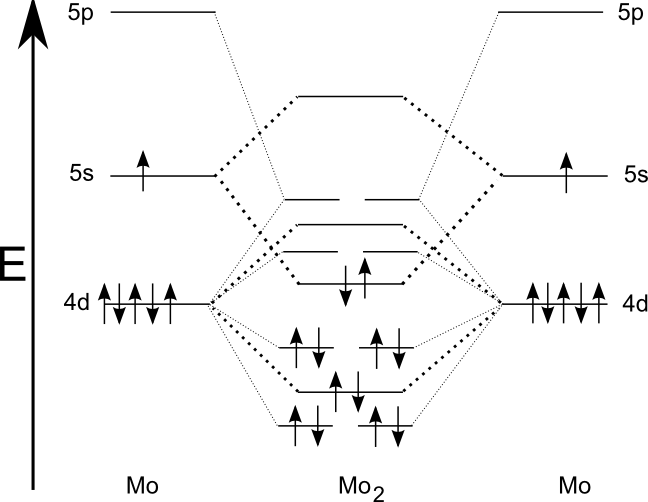
Sextuple Bond Wikipedia

Why Does Platinum S Electron Configuration Not Conform To Hund S Rule I E Have A Full 5d Orbital As Other Metals In Its Group Do E G Palladium Quora

The Bi Iii 6s Orbitals Are Visible At The Middle Points Of The Upper Download Scientific Diagram
Electron Configurations
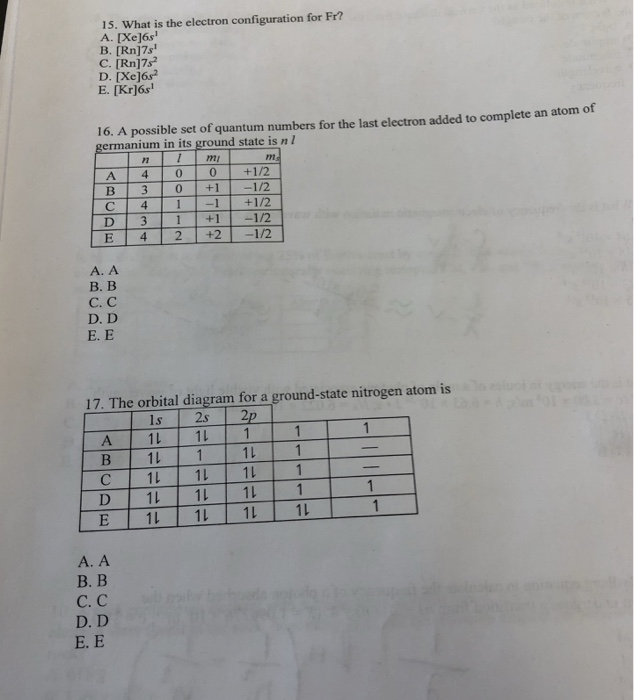
Solved 15 What Is The Electron Configuration For Fr B Chegg Com
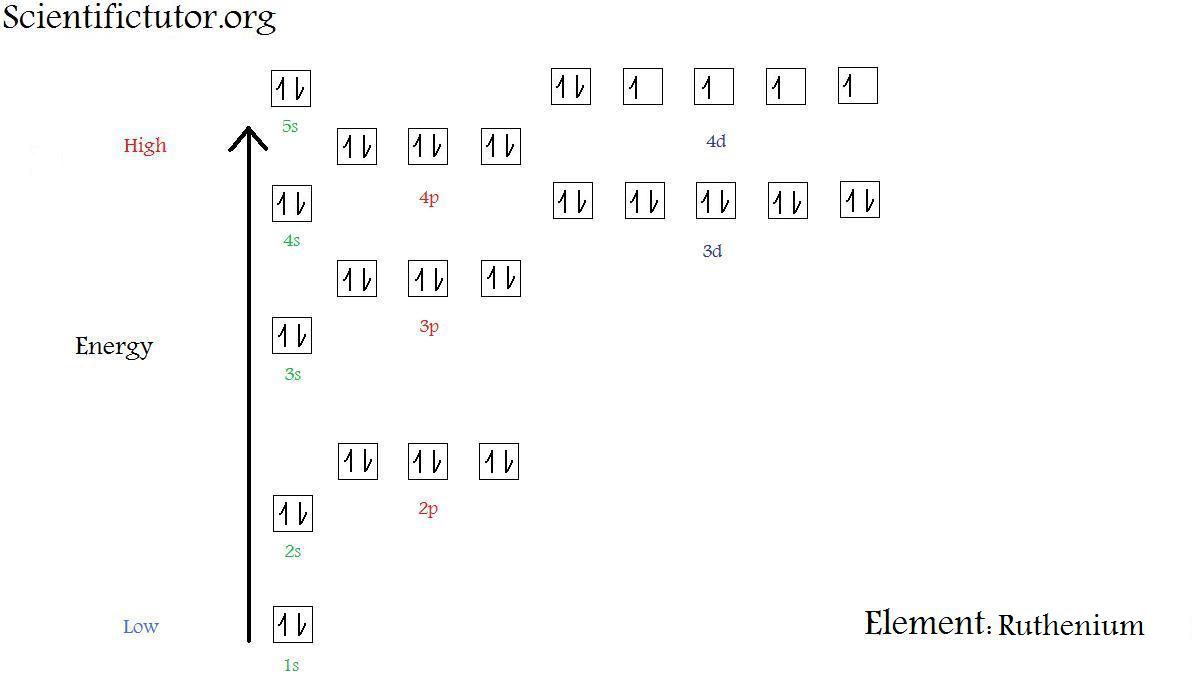
Chem Electron Configuration Diagrams Scientific Tutor
Electron Config
Clarifying Electron Configurations Chemical Education Xchange

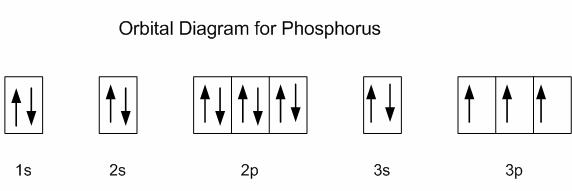
Build The Orbital Diagram For The Ion Most Likely Formed By Phosphorus Use The Buttons At Homeworklib
1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts
The Order Of Filling 3d And 4s Orbitals
1
Electronic Structure Of Atoms Electron Configurations Chemistry

Pdos On 2p Orbital Of C And 6s 5d Orbitals Of W 4c Atoms For Oc Vac Download Scientific Diagram
Scholarscompass Vcu Edu Cgi Viewcontent Cgi Article 1217 Context Phys Pubs
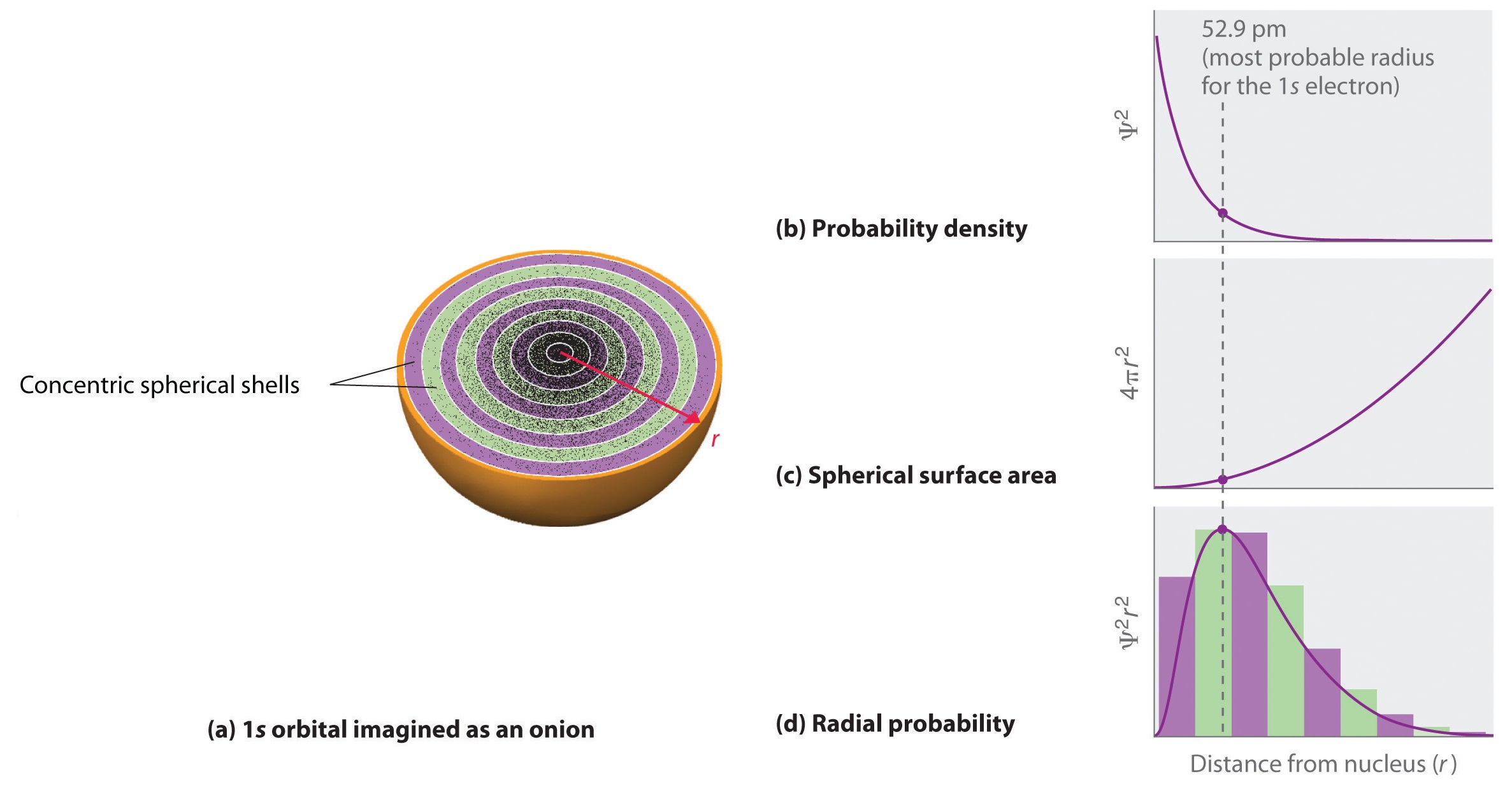
6 6 3d Representation Of Orbitals Chemistry Libretexts

11 29 16 Today I Will Determine The Probable Location Of Each Electron In An Atom Warm Up Explain Bohr S Electron Energy Levels Ppt Download
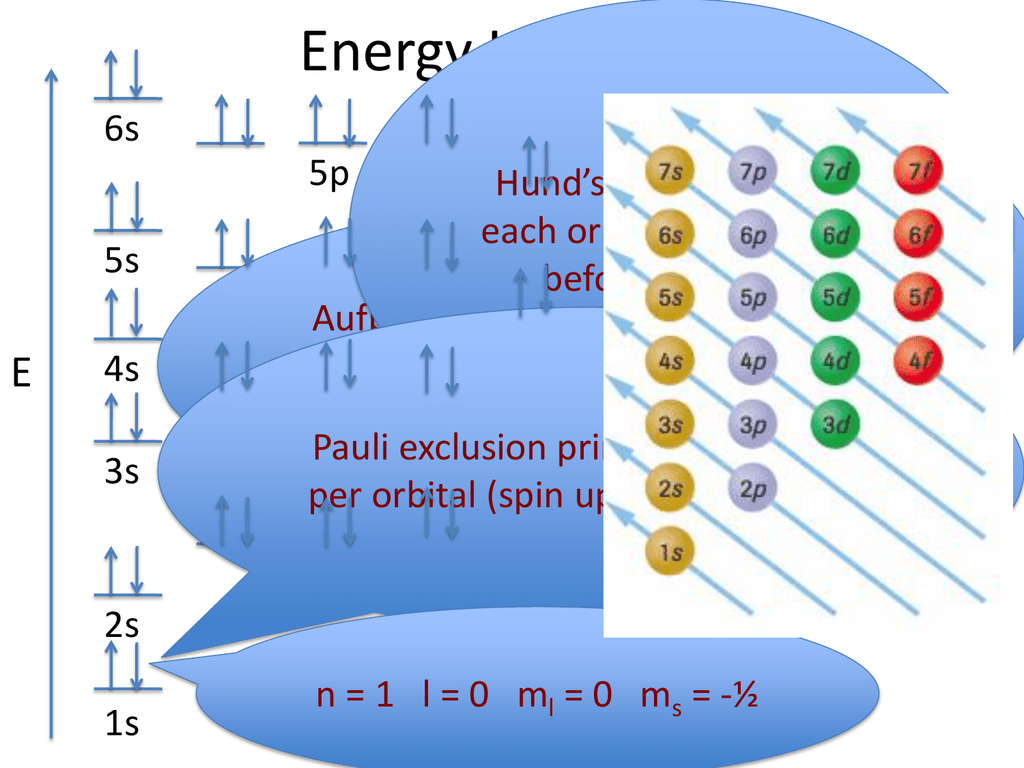
Electron Configuration

Orbital Diagrams Periodic Chart Method Of Filling Subshells Youtube

Figure 4 From Covalent Gold Semantic Scholar
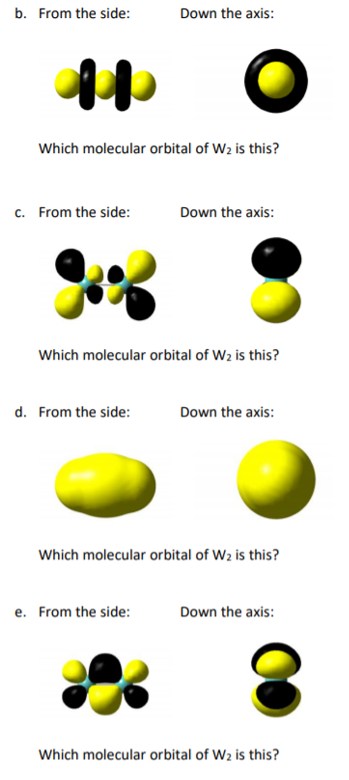
Solved The Diatomic Tungsten Molecule W2 Has The Follow Chegg Com

Solved Part A Enter The Orbital Diagram For The Ion Cd Chegg Com
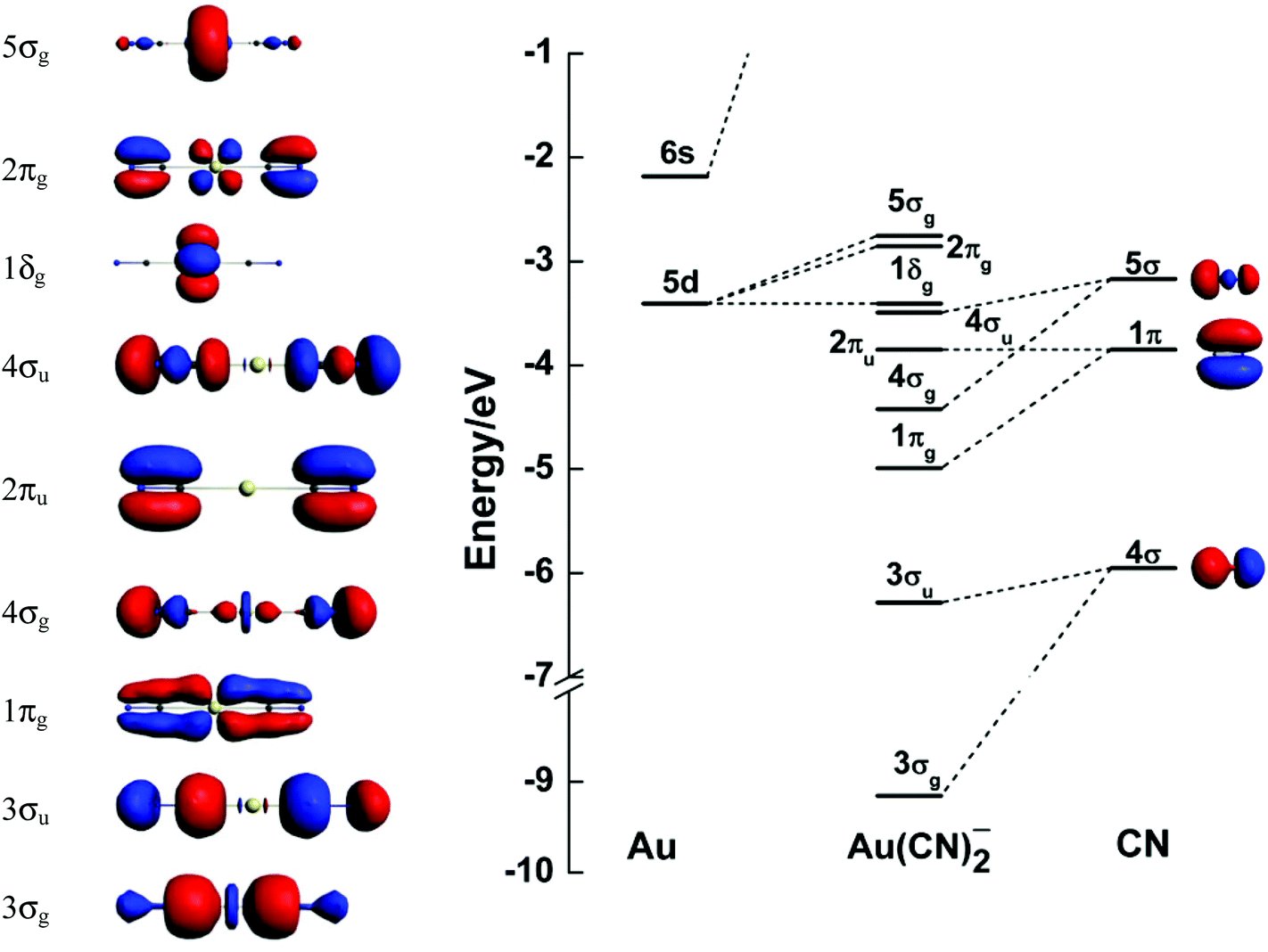
On The Gold Ligand Covalency In Linear Aux2 Complexes Dalton Transactions Rsc Publishing

Write Full Orbital Diagrams For Each Of The Following Elemen Clutch Prep
2a3 Orbitals
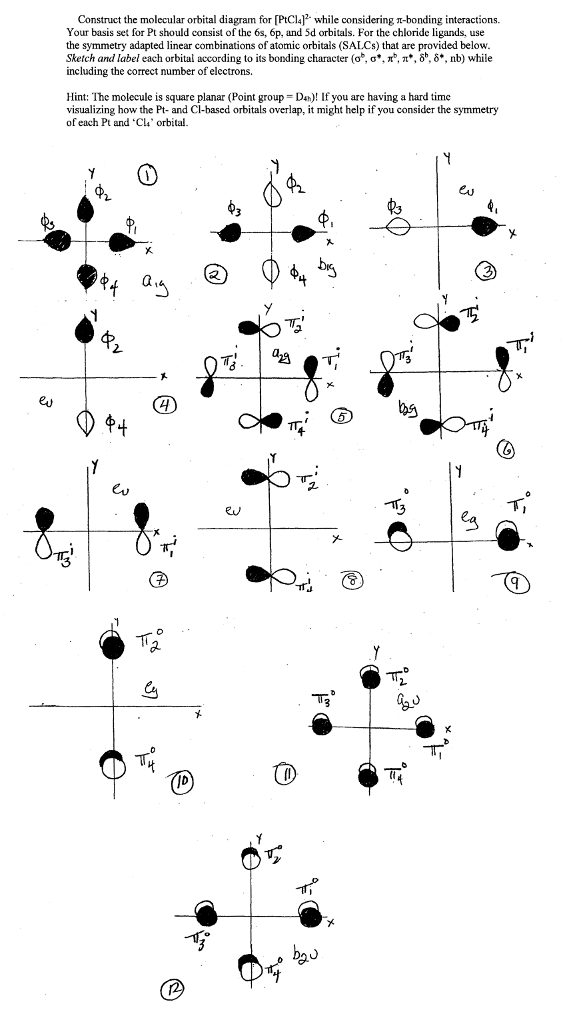
Solved Construct The Molecular Orbital Diagram For Ptcl4 Chegg Com

Color Online A The Pdos Of The Bi 6s Orbital Blue And Te 5p Download Scientific Diagram
Solved Chegg Com

Orbital Energy Diagram For Multi Electron Atom Study Chemistry Electron Configuration Chemistry
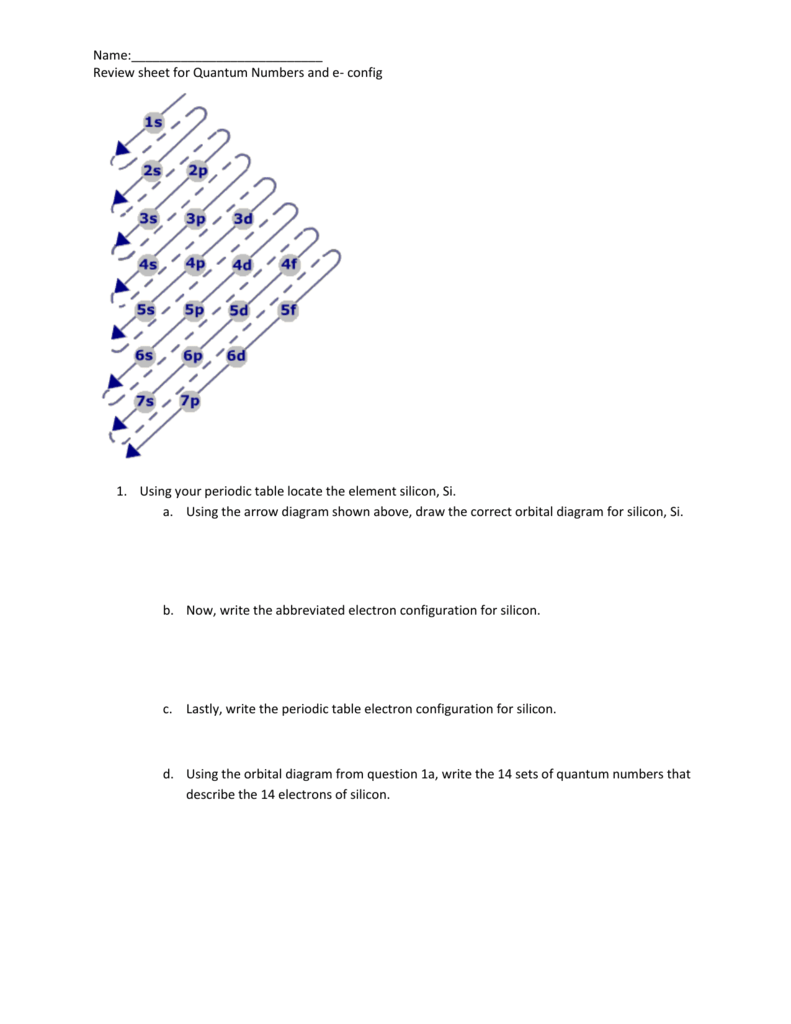
Unit 2 Quantum Numbers Review Packet
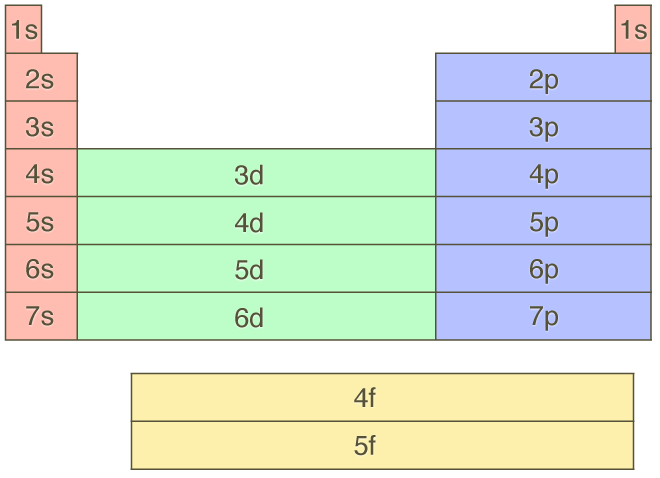
Aufbau Principle

The Calculated Homo And Lumo For The Free Pyridine Molecule And The 6s Download Scientific Diagram
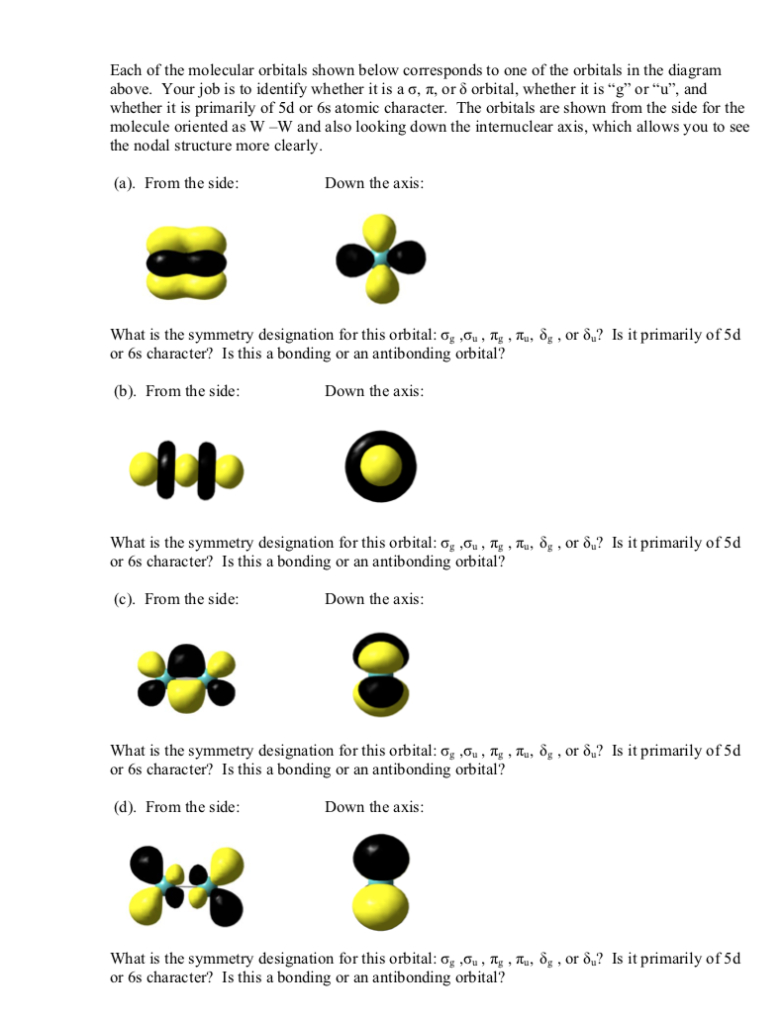
5 The Tungsten Dimer Molecule W2 Has The Molecu Chegg Com

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Figure 3 From Photoelectron Spectroscopy Of Biau And Bibo Further Evidence Of The Analogy Between Au And Boronyl Semantic Scholar
3 4 Electronic Structure Of Atoms Electron Configurations Chemistry Atoms First 2e Openstax

Electron Configurations
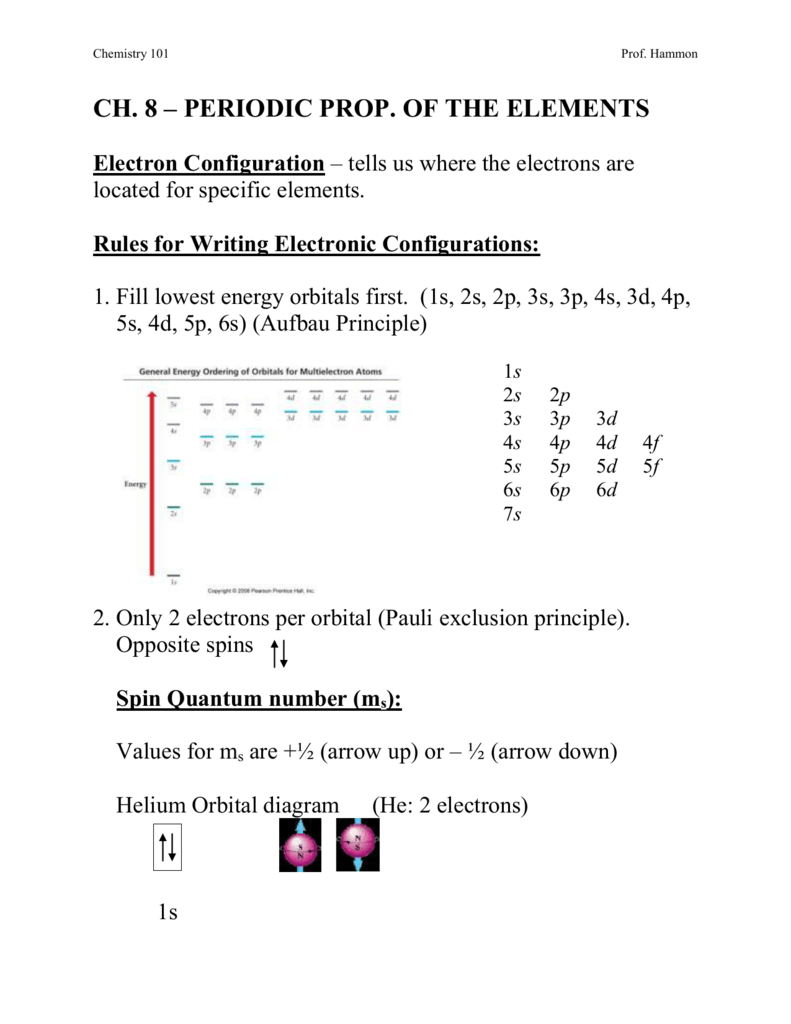
101ch8
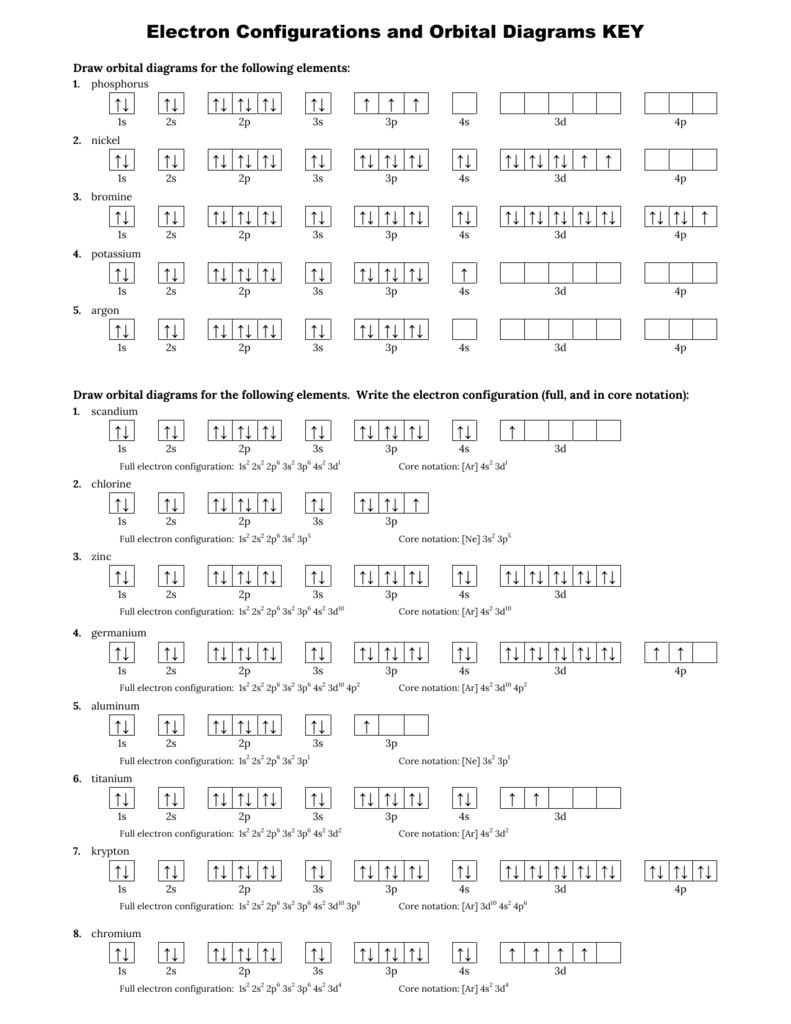
Electron Configurations And Orbital Diagrams Key
Draw The Orbital Diagram Associated With Each Of The Following Electron Configurations A 1 S 2 2 S 2 2 P 2 B 1 S 2 2 S 2 2 P 2

Quantum Numbers Atomic Orbitals And Electron Configurations
Many Electron Atoms The Electronic Basis Of The Periodic Table
Webelements Periodic Table Gold Properties Of Free Atoms

Solved A Two Types Of Nodes Occur In Atomic Orbitals Sp Chegg Com

Color Online A The Pdos Of The Bi 6s Orbital Blue And Te 5p Download Scientific Diagram

Electrons In Atoms 36 Electron Configuration Orbital Diagram And Quantum Numbers For W Youtube

Orbital Diagrams And Electron Configurations Vocabulary 1 Electron Configuration 2 Aufbau Principle 3 Pauli Exclusion Principle 4 Electron Spin 5 Hund S Ppt Download

3 Mer How Many Electrons In An Atom Can Have Each Of The Following Quantum Designations 2 Fourth Period And Forb Homeworklib

Aufbau Principle Wikipedia
Electronic Structure And Periodic Table Mcat Review
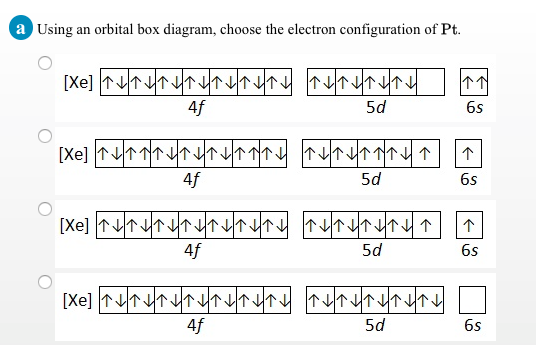
Solved Manganese Is Found As Mno2 In Deep Ocean Deposits Chegg Com

Solved The Diatomic Tungsten Molecule W2 Has The Follow Chegg Com



