D Orbital
Solved Sketch The Crystal Field D Orbital Splitting Diagr Chegg Com

Shapes Of Atomic Orbitals

Amazon Com Molecular Models 16 Do651 Platinum Series D Orbital Atomic Expansion Kit Grade 2 To 12 Pack Of 95 Industrial Scientific
Explain Shape Of D Orbitals Qs Study

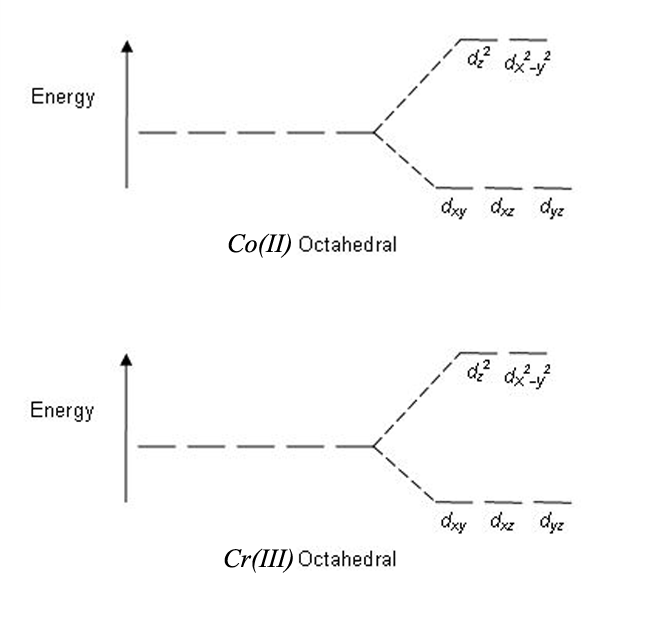
Crystal Field Theory Energy Level Splitting

Some Surprising Silicon Chemistry Chemical Connections
D-Orbital concentrates on providing the best daily timing signals for trading a variety of market sectors.

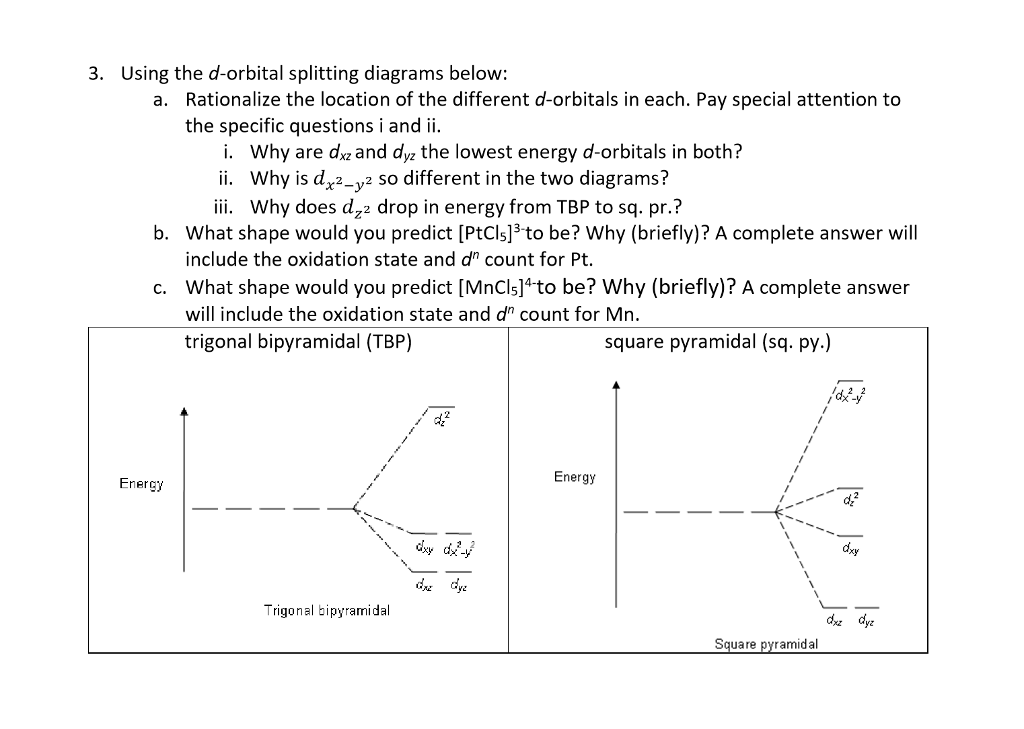
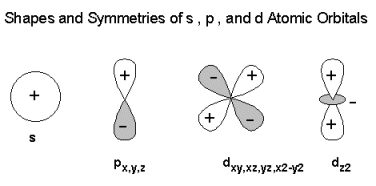
D orbital. This gives rise to five d orbitals, d xy, d yz, d xz, d x2-y2, and d z2. These orbitals are designated as d xy, d yz, d xz, d x2–y 2 and d z2. To describe the five bonding orbitals in a trigonal bipyramidal arrangement, we must use five of the valence shell atomic orbitals (the s orbital, the three p orbitals, and one of the d orbitals), which gives five sp 3 d hybrid orbitals.
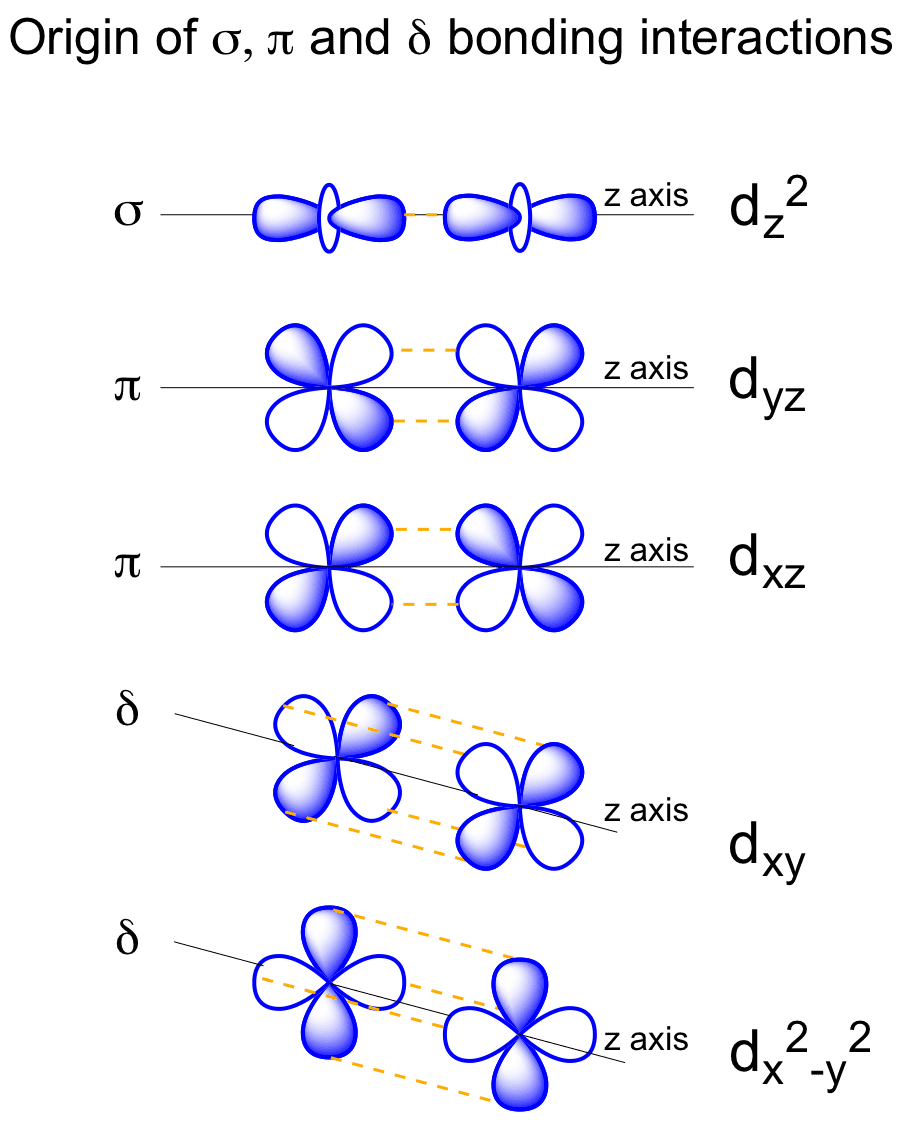
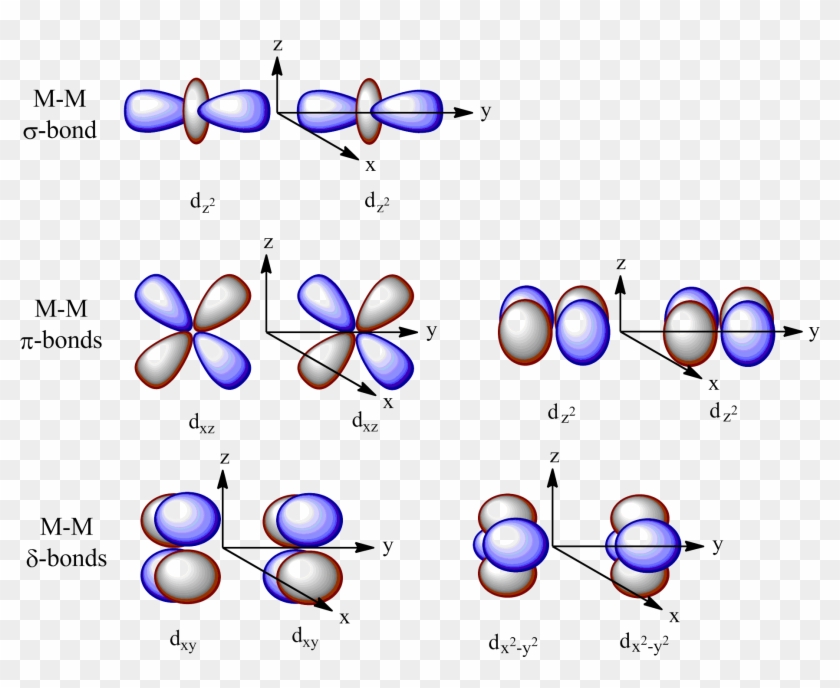
Both d xz and d yz can form π bonds with each other. Negatively charged electrons are attracted to a positively charged nucleus to form an atom or ion. Virtual Orbitals app helps you to visualize the shapes of the orbitals in 3D such that you can understand more and you can sort out your confusions.
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. How many d orbitals exist in one energy level (n ≥ 3) of an atom?. The orbital arrangement of an atom's electrons.
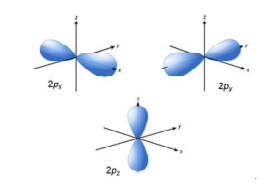
The overall total of 18 directional lobes point in every primary axis direction and between every pair. The orbital letters are associated with the angular momentum quantum number, which is assigned an integer value from 0 to 3. P orbitals are usually polar and form a teardrop petal shape with the point towards the nucleus.
(Actually, that turns out not to be true!. It has two lobes in the z-axis and a doughnut-shaped lobe in the xy plane. The Company is based in Como, Italy with subsidiaries in Lisbon, Portugal, Washington DC, and Harwell, UK.
Of these orbitals, d z 2 is unique;. Each additional electron usually goes into a 3d orbital. Each orbital is filled and the total is six electrons.
3D model to visualise the shapes of atomic orbitals. This is the 4th level of the tetrahedron. So if you're looking at the letter designation "D," a D orbital holds 2 electrons but the D subset holds 10 electrons.
A SUBSET, however, is all of the boxes for whatever letter designation you're talking about. Imaging features of these lesions often reflect their tissue composition. So that is 2pz and the orbitals keep going.
That’s not a thing. The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2). D-block elements are thought of as elements in which the last electron to be added to the atom is in a d orbital (actually, that turns out not to be true!.
Some people would call that 2py. He describes it as a variation in energetic sequence from group 1 to 3 elements such that the order changes from 4s<4p<3d in K, 4s<3d<4p in Ca and 3d<4s<4p for Sc and subsequent elements. Notice that the 1s orbital has the highest probability.
This you could view as the in and out of the page so you could view that as the z-dimension. D Orbital The d orbital contains 10 electrons. This education app help the students to learn chemistry in a smarter way.
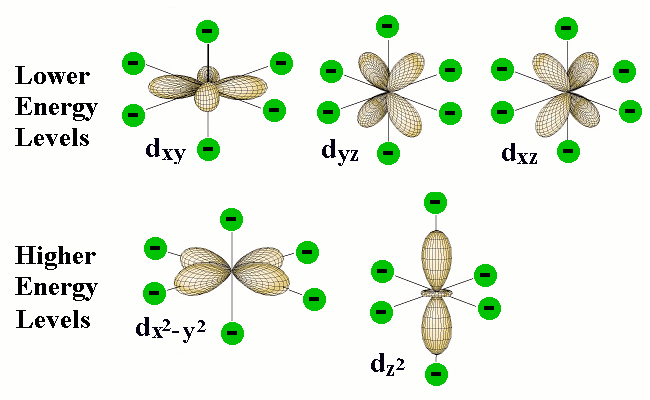
Explanation of Degenerate Orbitals with Diagram. The other two d orbitals are at higher energy due to the crystal field of the ligands. Thus d orbital corresponds to 4 double dumb-belled shapes (d xy, d yz, d zx, d x 2 y 2) with the atomic nucleus at its centre and one dumb belled with dough nut shaped (d z 2).
In developing its programs, d-Orbital acts much like the master chef. The p orbitals are often referred to as being. There are five d orbitals:.
The d orbital has ten protons+ Read More. The formalism has been incorporated into the two major models used to describe coordination complexes;. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental).
Six electrons would be written 3d 6 ;. ℓ = 2 orbital is called a d orbital. 2) Orbitals are combined when bonds form between atoms in a molecule.
D-Orbit is dedicated to making its business a force for good, optimizing both economic performance and social impact in order to improve the environmental capital. RMetx™, a pure solution of zinc and silver minerals in water, is a dietary supplement that supports immune health.rMetx™ provides zinc in a bioactive soluble solution, which can support immune system activity.*. Sp 3 d and sp 3 d 2 Hybridization.
Hence d orbitals have five orientations in space. How to use orbital in a sentence. Cavernous malformations (also known as cavernous hemangiomas), although not true neoplasms, are the most common benign adult orbital ….
There is one type of s orbital, which is spherically shaped. Remember an orbital is just one box when you're drawing your orbital diagram. Orbital neoplasms in adults may be categorized on the basis of location and histologic type.
The value of mathn-1/math is the maximum value of the quantum numbermath l/math, so if you have a d orbital, mathl/math is 2. • Computers would prefer to work with 6 d functions (dx2, dy 2, dz, dxy, dxz, and dyz);. Set of five ‘d’ Orbital Models, Includes one each of (KS9009)dxy, (KS9011)dzx, (KS9012)dx2, (KS9010)dy2, and (KS9008)dz2.
For d orbital Azimuthal quantum number l = 2 and the magnetic quantum number m = -2, -1, 0, +1, +2. Note that the 3s and 3p protons are not shown in this tetrahedral view, but are addressed in section on nucleus structure. For the d-orbital, the magnetic quantum number m l can equal -2 to 2, taking the possible values -2, -1, 0, 1, or 2.
D-Orbit places equal emphasis on three pillars:. What Does S, P, D, F Stand For?. Which choice ranks the given orbitals in order of increasing size?.
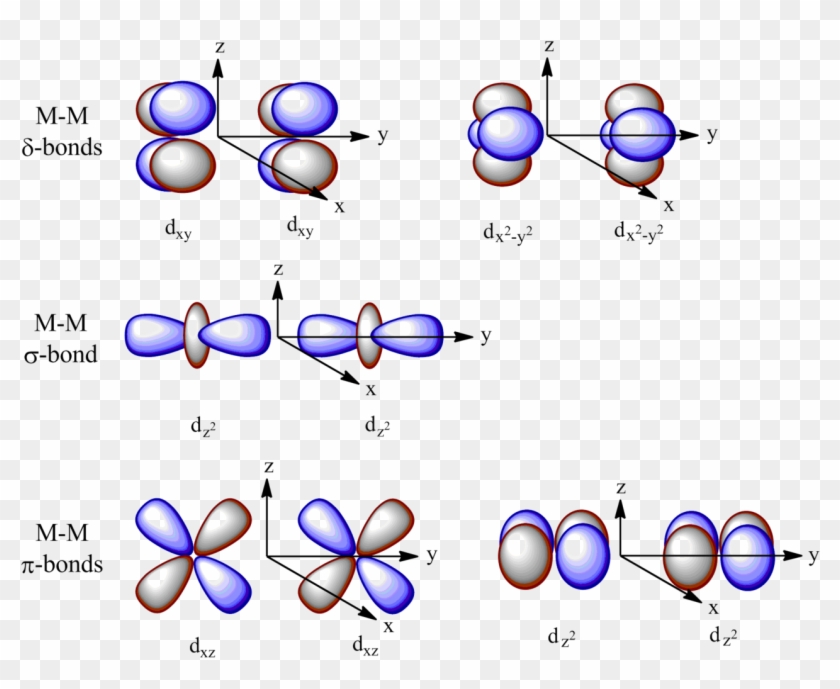
While we strive to produce cutting-edge, analytical, rigorous, adaptive models, our ultimate goal is to provide the advisor with a total solution. And a d subshell (l = 2) consists of five orbitals, called d orbitals. These diagrams show the origin of σ π and δ bonding between two d orbitals aligned along the z axis.
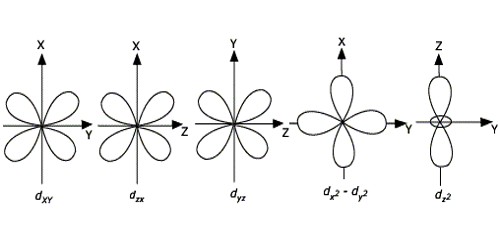
There are three types of p orbitals, referred to as p x, p y, and p z. The fifth and final d-orbital consists of three regions of high probability density:. We are the first aerospace company to have received the B-Corp certification worldwide.
These orbitals are similar to the p orbital shape, but with more 'petals' like a cloverleaf. This is why the hydrogen atom has an electron configuration of 1s 1. Remember that in an isolated atom or ion, the five d orbitals all have the same energy - they are said to be degenerate.
Before we can use these orbitals we need to know the number of electrons that can occupy an orbital and how they can be distinguished from one another. When angular quantum number l=2, it is considered the d-orbital. Hence, we can say that there are five d-orbitals.
This picture is consistent with the experimental fact that the complex is diamagnetic, meaning that it has no unpaired electrons. The electric fields associated with the ligands cause repulsions in the d orbitals and that raises their energies. An orbital is the region of space around the nucleus within which the probability of finding an electron of given energy is maximum.The shape of this region (electron cloud) gives the shape of the orbital.
Once principle quantum number n equals 3 or greater, angular quantum number can equal 2. The number of orbitals in a subshell is therefore 2 (l) + 1. These are called “6 Cartesian d functions” • dx 2+ dy + dz looks like an s orbital • Similar answers are obtained using 5 or 6 d functions • For f functions, it’s 7 versus 10 f functions • Common reason for disagreement between calculated.
Explanation of D orbital. Orbitale definition, the lowermost point on the lower margin of the left orbit, located instrumentally on the skull or by palpation on the head. Orbitals in the 2p sublevel are degenerate orbitals – Which means that the 2px, 2py, and 2pz orbitals have the exact same energy, as illustrated in the diagram provided below.
This is illustrated in the figure below. We will come back to that in detail later.) The electronic structures of the d-block elements are shown in the table below. The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2).
That means the lowest value for n must be 3. D orbitals start from the 3rd shell and their wave functions are mentioned below. It is impossible to learn about the shapes of orbitals in a page which is 2D but the orbitals aren't 2D.
They can also have ring shapes around the base of the petals. Orbital d (l = 2) má tvar složitější, členěný do více laloků, se vzájemně tvarově odlišnými formami. D z 2 is capable of forming a σ interaction with another d z 2.
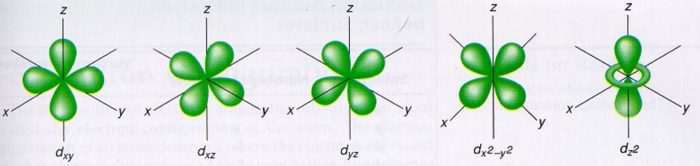
Prostorové formy se označují d xy , d xz , d yz , d x ²- y ² , d z ². Profit, benefit, global impact. This could be the 2p orbital that is in the y-dimension as some people call that 2px.
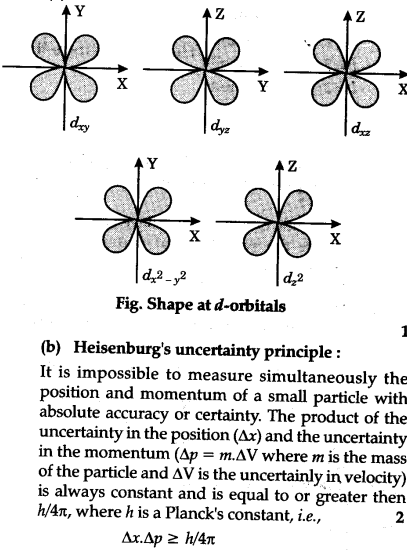
That changes when ligands are attached. D xy, d xz, d yz, d x 2-y 2, d z 2 When one electron is accommodated by the 3d orbitals it is written 3d 1 ;. The individual orbitals are labeled with the magnetic quantum number, m l , which can take the 2 l + 1 values l , l − 1,…, − l.
An orbital is defined as a region in an atom where there is a high probability of finding an electron. The s correlates to 0, p to 1, d to 2, and f to 3. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes.
The s, p, and d orbitals will be introduced in this section. Founded in 11, D-Orbit currently employs approximately 80 people. The d orbitals are named in relation to the x, y, and z axes.
While adhering to an exacting scientific methodology and industry standard tools, creativity is used to add a certain je nais se quoi. The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. Here we provide a concise summary of the key features of orbital splitting diagrams for square planar complexes, which we propose may be used as an updated reference in chemical education.
D x 2-y 2 and d xy can interact with each other to form δ bonds. That’s why when you look at. D-block elements are thought of as elements in which the last electron to be added to the atom is in a d orbital.
S, p and d. The second, ℓ = 1 is called a p orbital. Out of these five d orbitals, shapes of the first four d-orbitals are related to each other, which is different from the dz2 orbital whereas the energy of all five d orbitals is the same.
Ten electrons would be written 3d 10. Which orbital is indicated by the quantum numbers n = 3, l = 2, ml = −1?. There is a d-orbital once you get to the third shell.
Note that unpairing happens in excited states, wehn energy must be put in to put the electrons on a higher energy state. The angular momentum quantum number can be used to give the shapes of the electronic orbitals. D z 2, d xz, d yz, d xy, and d x 2 − y 2.
When I was perusing the works of Schwarz on atomic structure, I came across the unfamiliar term of d-orbital collapse. Each orbital can only hold 2 electrons. I have no idea.
Students can see the every parts of orbitals by rotating their. As a result, d-Orbital's solutions will invariably be different from all other available solutions as we strive to satisfy even the most discerning investor. Similarly, the 3px, 3py, and 3pz are degenerate orbitals.
Orbital definition is - of, relating to, or forming an orbit (such as the orbit of a moon, planet, or spacecraft). The presentation of d-orbital splitting diagrams for square planar transition metal complexes in textbooks and educational materials is often inconsistent and therefore confusing for students. We will come back to that in detail later.) The electronic structures of the d-block elements are shown in the table below.
Same with chlorine, which gives it a maximum valency of 7. Orbital, in chemistry and physics, a mathematical expression, called a wave function, that describes properties characteristic of no more than two electrons in the vicinity of an atomic nucleus or of a system of nuclei as in a molecule. There is one orbital in an s subshell (l = 0), three orbitals in a p subshell (l = 1), and five orbitals in a d subshell (l = 2).
Protons forming in nucleus.
.jpg)
Comparing The Logic Behind The Spdf And Mcas Models
Solved 3 Using The D Orbital Splitting Diagrams Below R Chegg Com
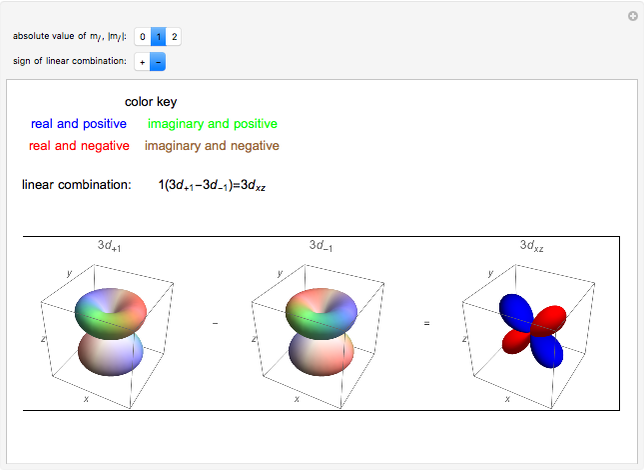
Linear Combinations Of D Orbitals Wolfram Demonstrations Project

Structure Reactivity Atoms
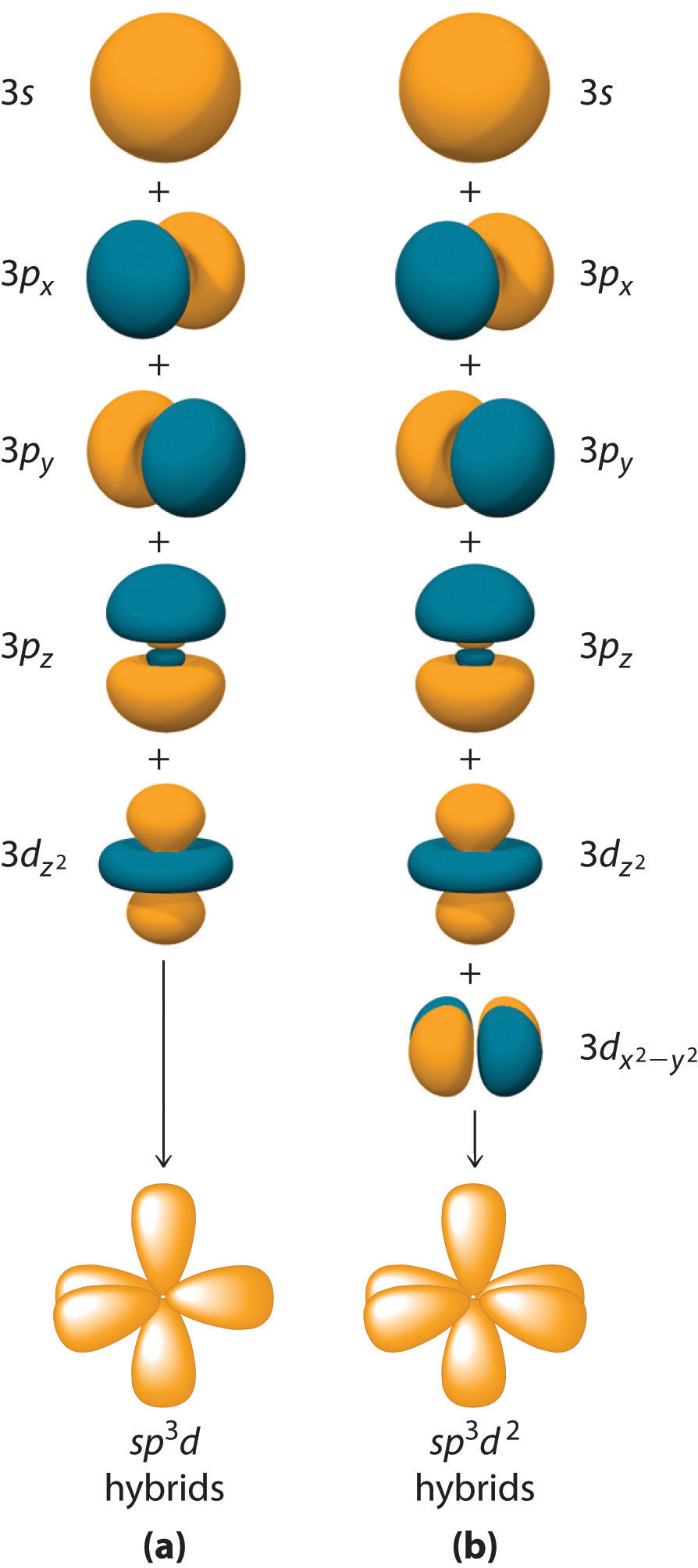
4 6 Hybridization Using D Orbitals Chemistry Libretexts

Orbit Subshell Orbital Electron

How To Draw All 5 D Orbitals Quora
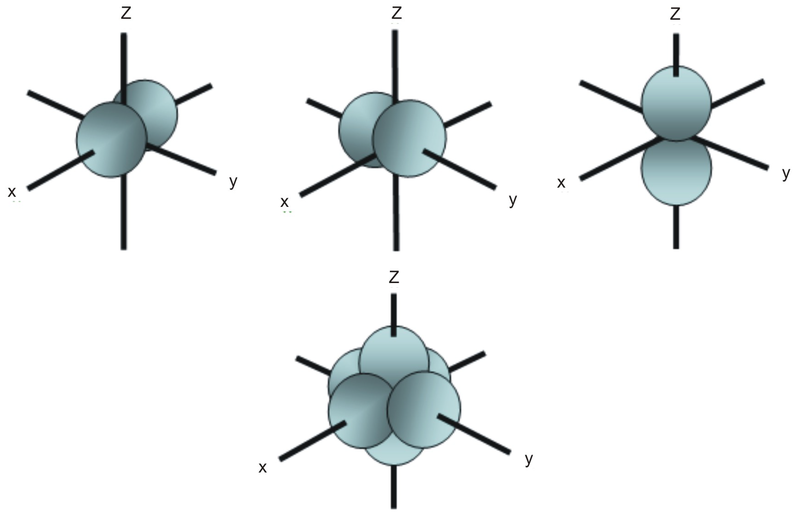
S P D F Orbitals Chemistry Socratic
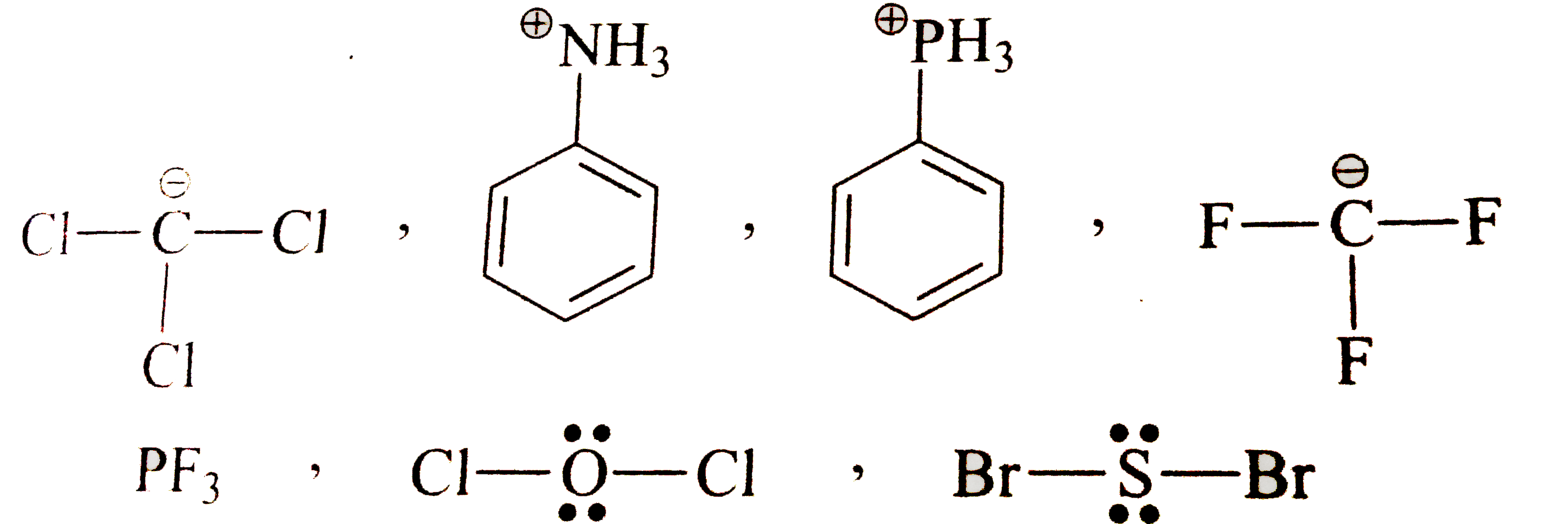
How Many Compounds From Following Exhibit D Orbital Resonance Br

What Do S P D Orbitals Look Like Visualizing Orbitals Using Computational Chemistry Part Two Youtube

Five D Orbitals In A Cubic Crystal Field Which Split Into Two E G Download Scientific Diagram

Shapes Of Atomic Orbitals Definition Examples Diagrams
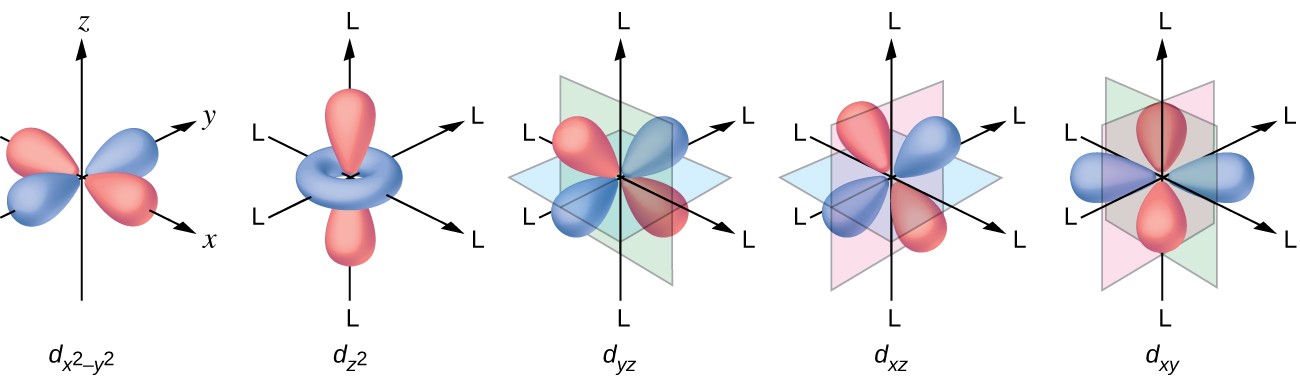
How Many Orbitals Are Found In A D Subshell Socratic
Q Tbn 3aand9gcqulzhcplpqcomqengxsfh8zlrg Jw3 8b 3am6v0f U Tzuel Usqp Cau

Introduction To Orbitals Stahl 9 12
Shapes Of Atomic Orbital Chemistry Class 11 Structure Of Atom

Nickel Ii Loses Electrons From 4s Which D Orbital Electrons Are Moved To Higher D Orbitals By Absorbing Visible Light And Why Isn T Nickel Ii 3d8 Mcat
Q Tbn 3aand9gcqulzhcplpqcomqengxsfh8zlrg Jw3 8b 3am6v0f U Tzuel Usqp Cau

A Schematic Representation Of The D Orbital Splitting And P D Download Scientific Diagram

D Orbital Shape Ewt

Color Online The D Orbital Energy Splittings Of A Transition Metal Download Scientific Diagram
What Is The Shape Of A D Orbital Quora

D Orbitals From Eric Weisstein S World Of Chemistry

Magnetic Quantum Number Chemistrygod

Draw The Shapes Of Five D Orbitals

Science Skool Atomic Orbitals
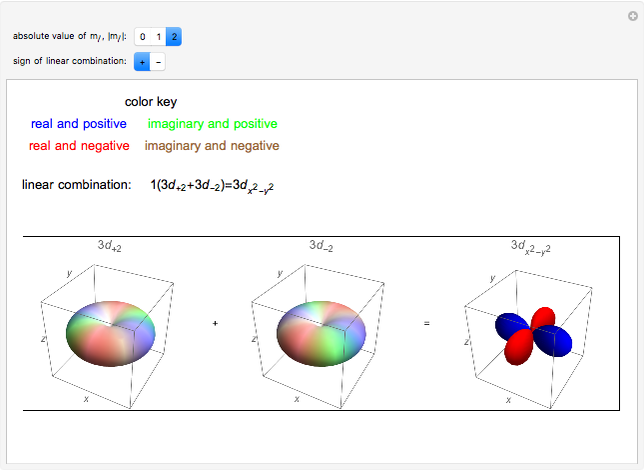
Linear Combinations Of D Orbitals Wolfram Demonstrations Project

Five D Orbitals In A Cubic Crystal Field Which Split Into Two E G Download Scientific Diagram
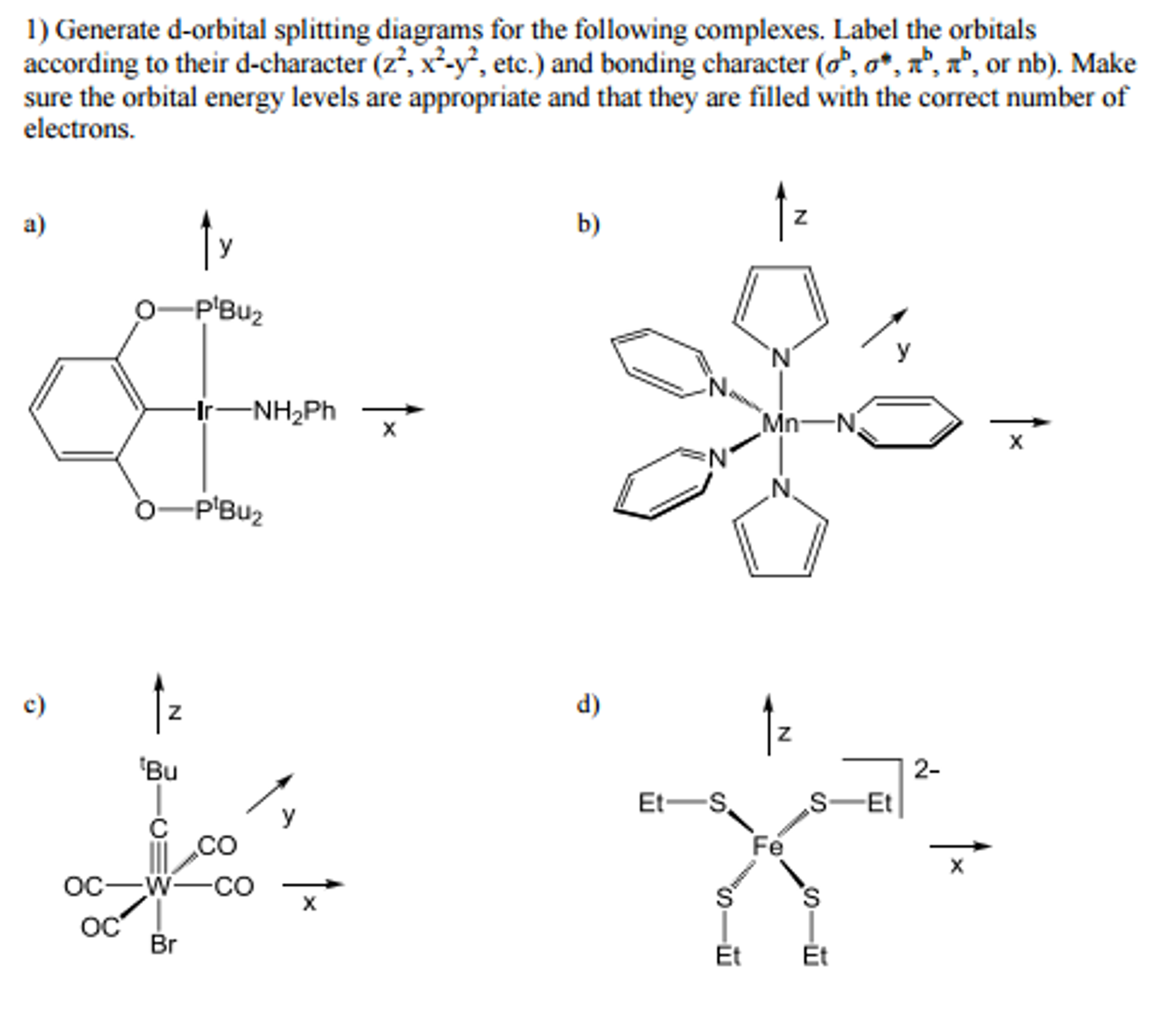
Generate D Orbital Splitting Diagrams For The Foll Chegg Com
What Do You Mean By Axial And Nonaxial Orbitals Quora

What Is D Orbital Collapse Chemistry Stack Exchange
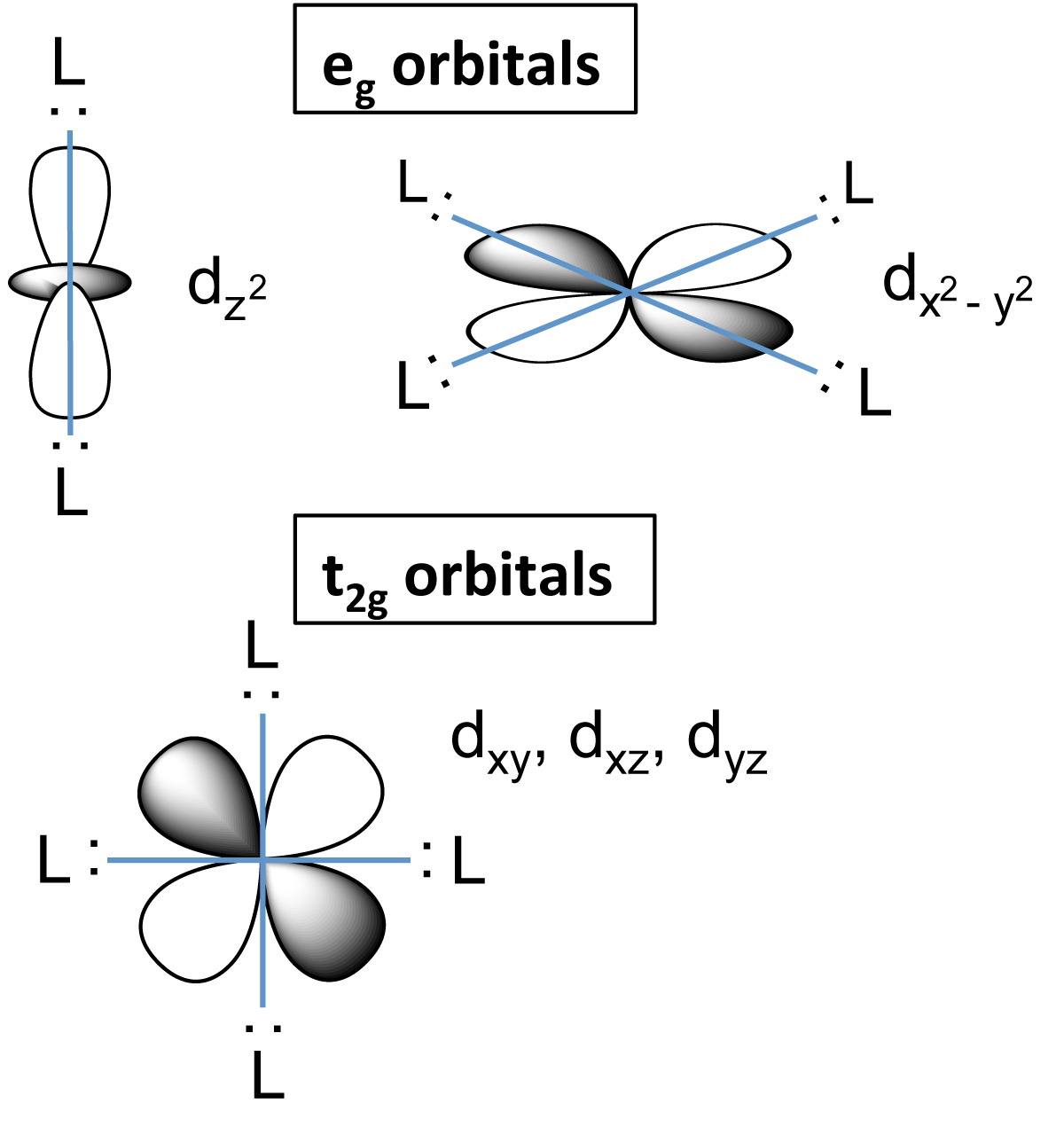
File D Orbital Splitting Png Wikimedia Commons

The Five D Orbitals Lose Their Degeneracy By An Octahedral Arrangement Download Scientific Diagram

Quantum Number Wikipedia
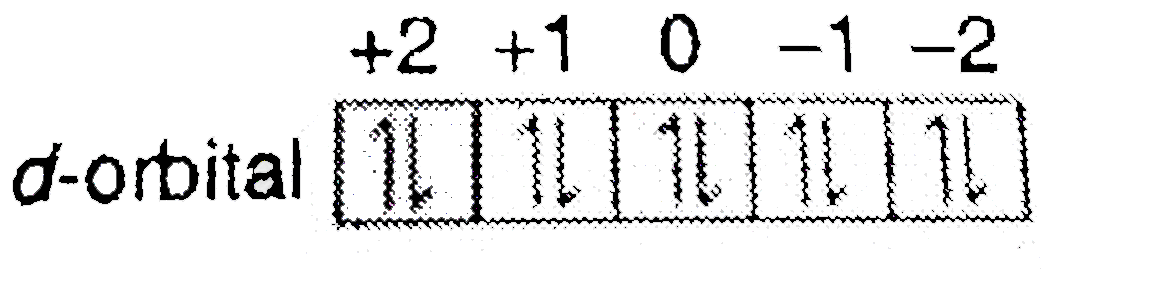
The Maximum Number Of Electrons That D Orbital Can Contain Is
How Do S P And D Orbitals Differ Socratic
9 9 Bonding In Coordination Complexes Chemistry Libretexts
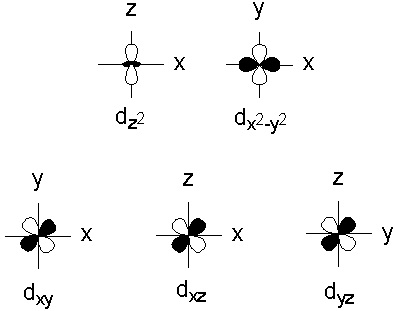
D Orbitals
Salc D D Orbital Overlap

D Orbital Shape Ewt
Orbitals And Their Types S P D F Orbitals And Their Shapes

F Orbital Shape Ewt

Representations Of Orbitals
Draw The Shapes Of D Orbitals Cbse Class 11 Chemistry Learn Cbse Forum
Bonding For Uv Visible Absorption Spectrometry D Orbital Diagram Free Transparent Png Clipart Images Download
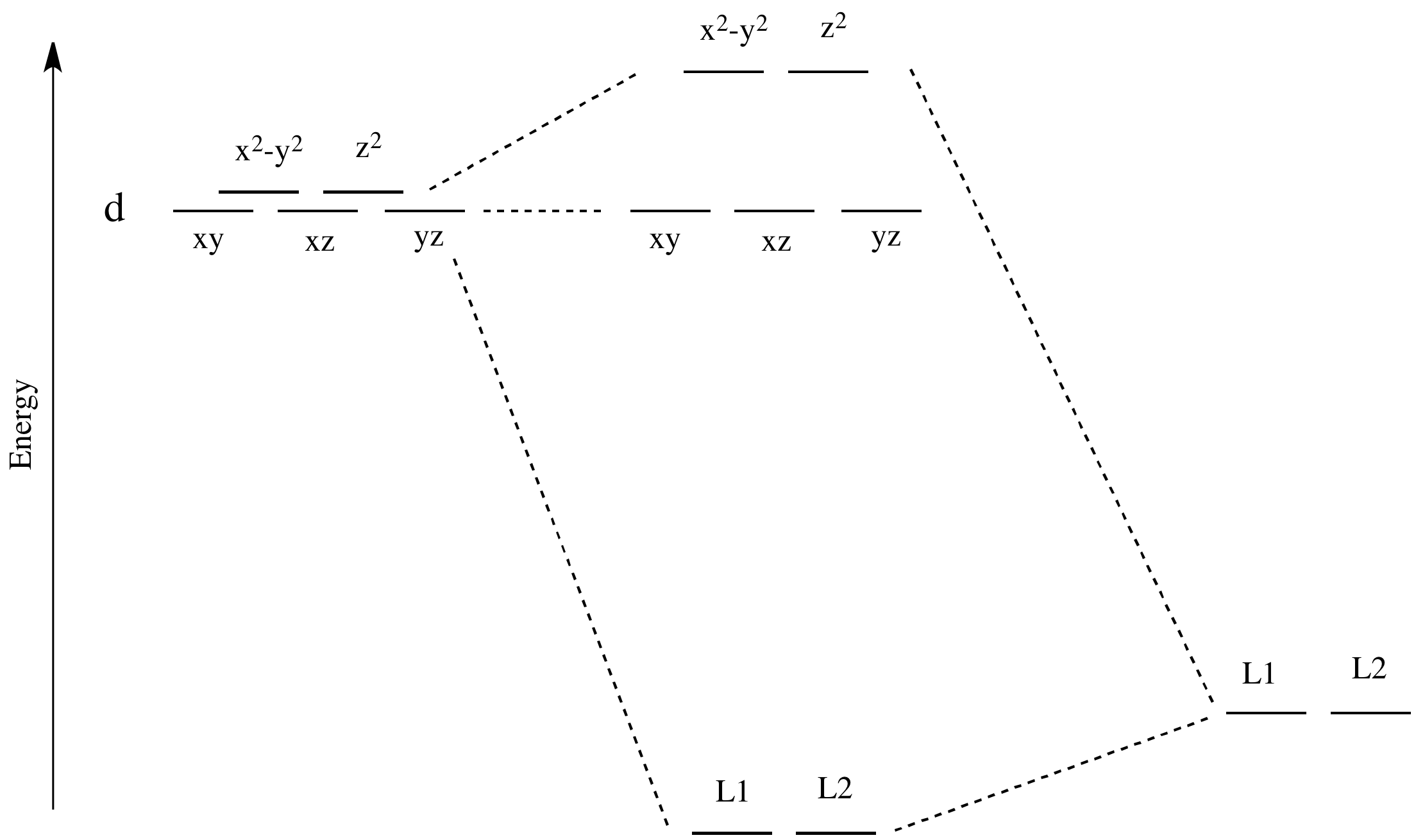
Coordination Chemistry
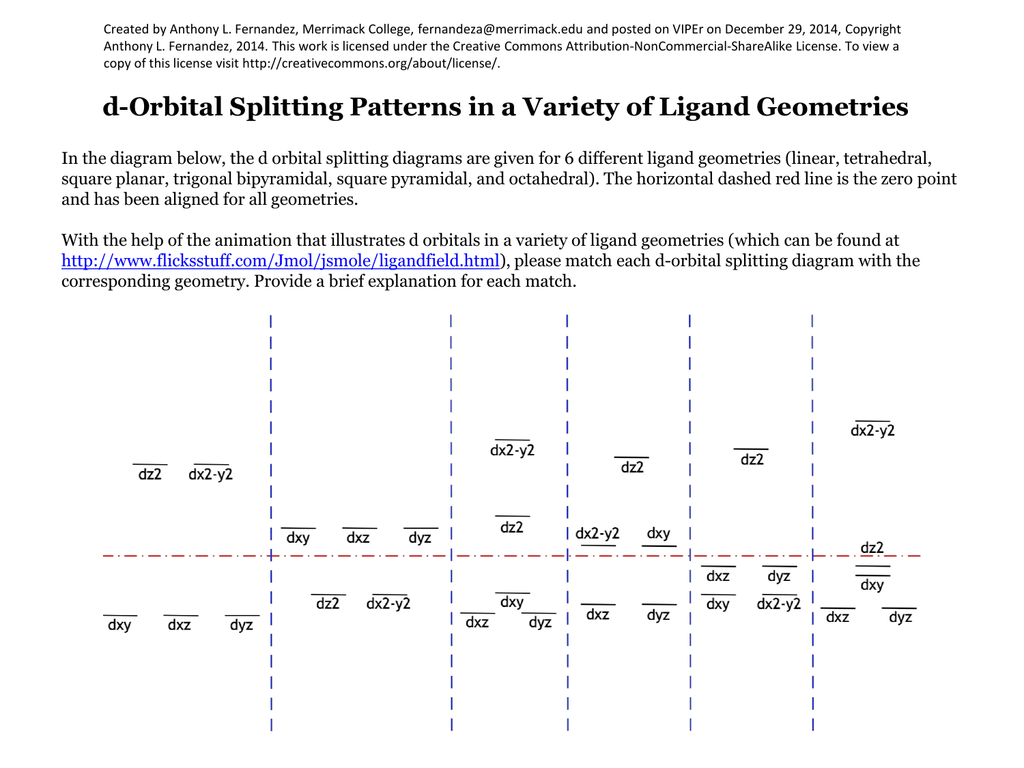
In The Diagram Below The D Orbital Splitting Diagrams Are Given For 6

Angular Probability Distribution Of D Orbital Study Chemistry Physical Chemistry Online Study

D Orbital Resonance And Dancing Sigma Bond Resonance Hindi General Organic Chemistry Pre Medical Exams Unacademy

D Orbital Physics Britannica

Why The Opposite Lobes Of P Orbitals Have Different Signs And Why The Opposite Lobes Of D Orbitals Have Chemistry Structure Of Atom Meritnation Com
Linear Combination Of Atomic Orbitals Wikipedia D Orbital Diagram Free Transparent Png Clipart Images Download

D Orbital Physics Britannica

Chemistry

13 9 Effects Of Different Ligands On Splitting Of The D Orbitals In Transition Metals Kerem S Chemistry Notes Ib

Atomic Orbitals Chemistry Topics
D Orbital Splitting For Different Geometries Youtube
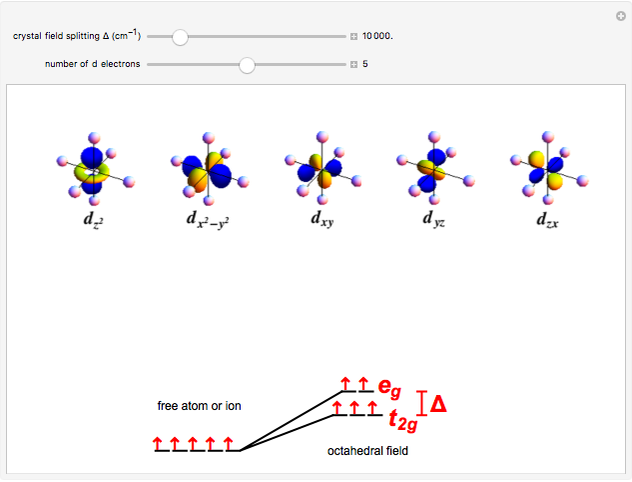
D Orbitals In An Octahedral Field Wolfram Demonstrations Project

Considering The Shape Of The P And D Orbitals Won T The Electrons Collide With The Nucleus At The Center Quora
Q Tbn 3aand9gctumdkhxhhko9x Vt1iug86adydqjyfpyc Tvwesklbjwhad8pz Usqp Cau

Atomic Orbital Chemistrygod

Chemistry D Orbitals

Chemical Bonding Shapes Of Atomic Orbitals Britannica
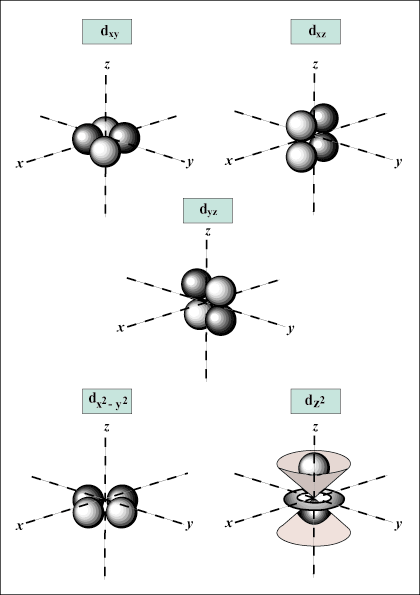
Atomic Structure Atoms And Atomic Orbitals Sparknotes
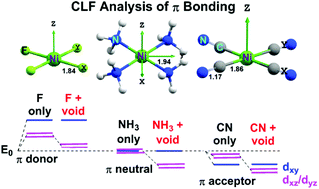
D Orbital Energy Levels In Planar Miif4 2 Mii Nh3 4 2 And Mii Cn 4 2 Complexes The Nature Of M L P Bonding And The Implications For Ligand Field Theory Dalton Transactions Rsc Publishing

I Think The Answer Is B As Phosphorus Has Vacant D Orbitals And It Can Use It To Accept Electron From Carbon But Can It Not Be Chlorine Since It Also Has
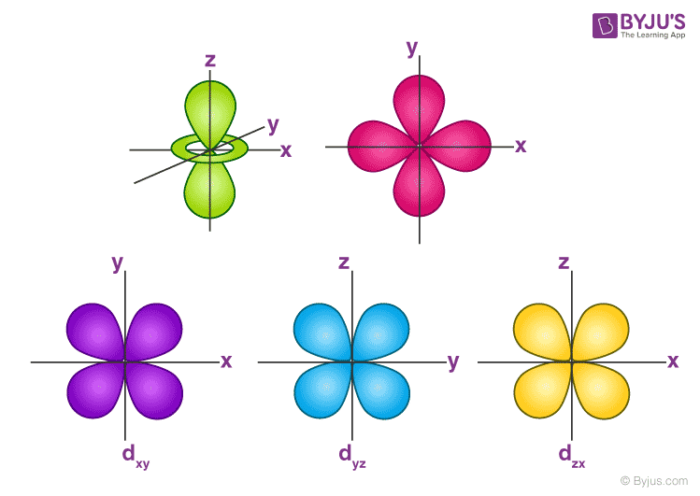
Orbitals Chemistry Shapes Of Atomic Orbitals Shape Of S P D And F Orbital

Reordering D Orbital Energies Of Single Site Catalysts For Co2 Electroreduction Han 19 Angewandte Chemie Wiley Online Library

S P D F Obitals Notation Shapes Diagrams How To Work Out Electron Arrangements Configurations Order Of Filling Quantum Levels Electronic Structure Of Atoms Gce A Level Revision Notes
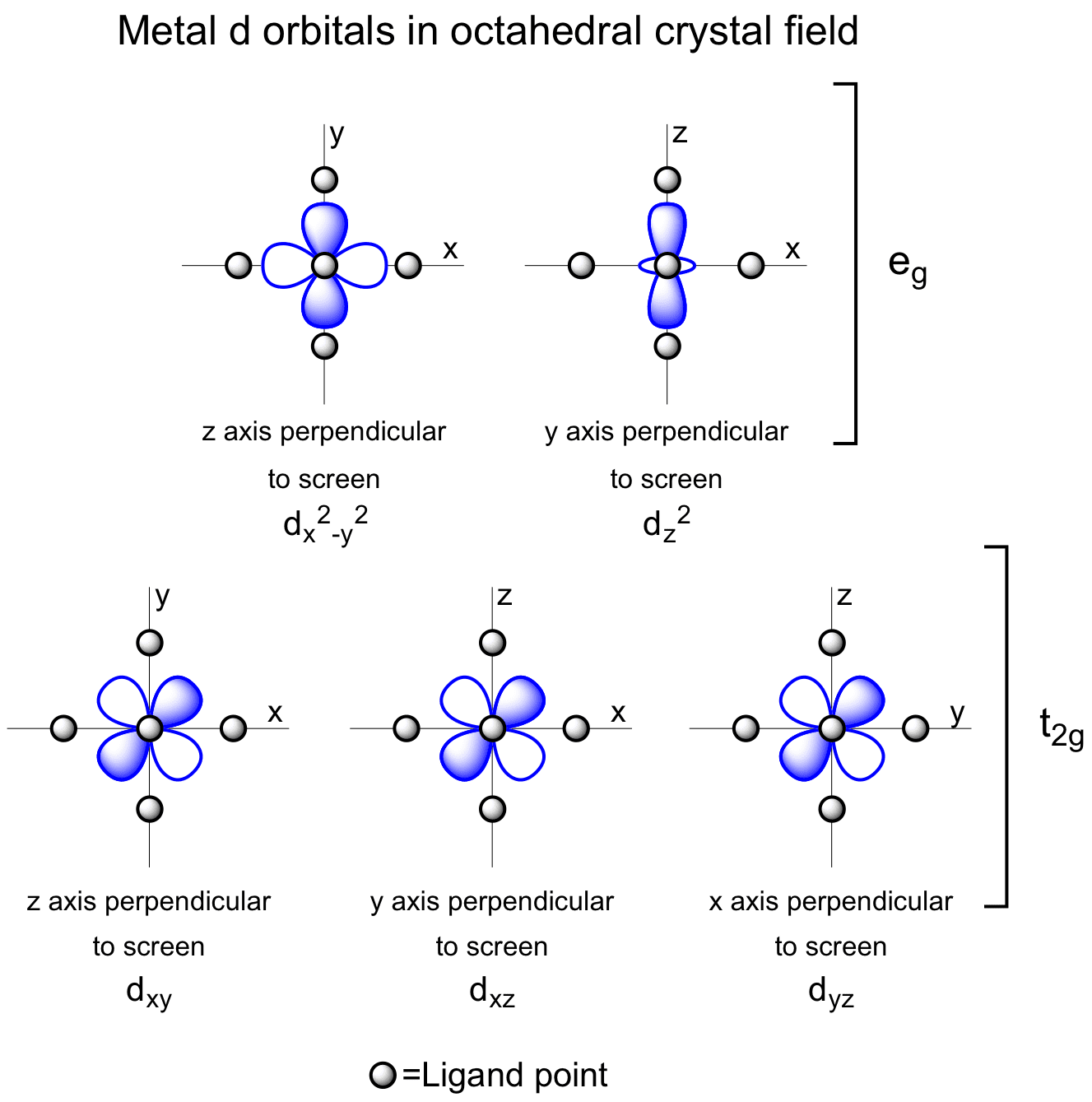
Metal D Orbitals In An Octahedral Crystal Field
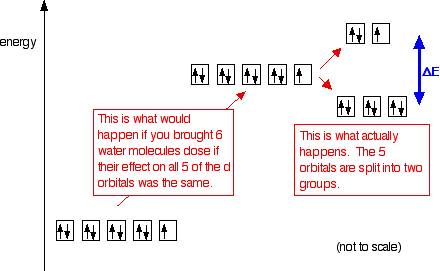
Complex Ions More About D Orbitals
Shape Of D Orbitals Chemistry Guider Swap Youtube
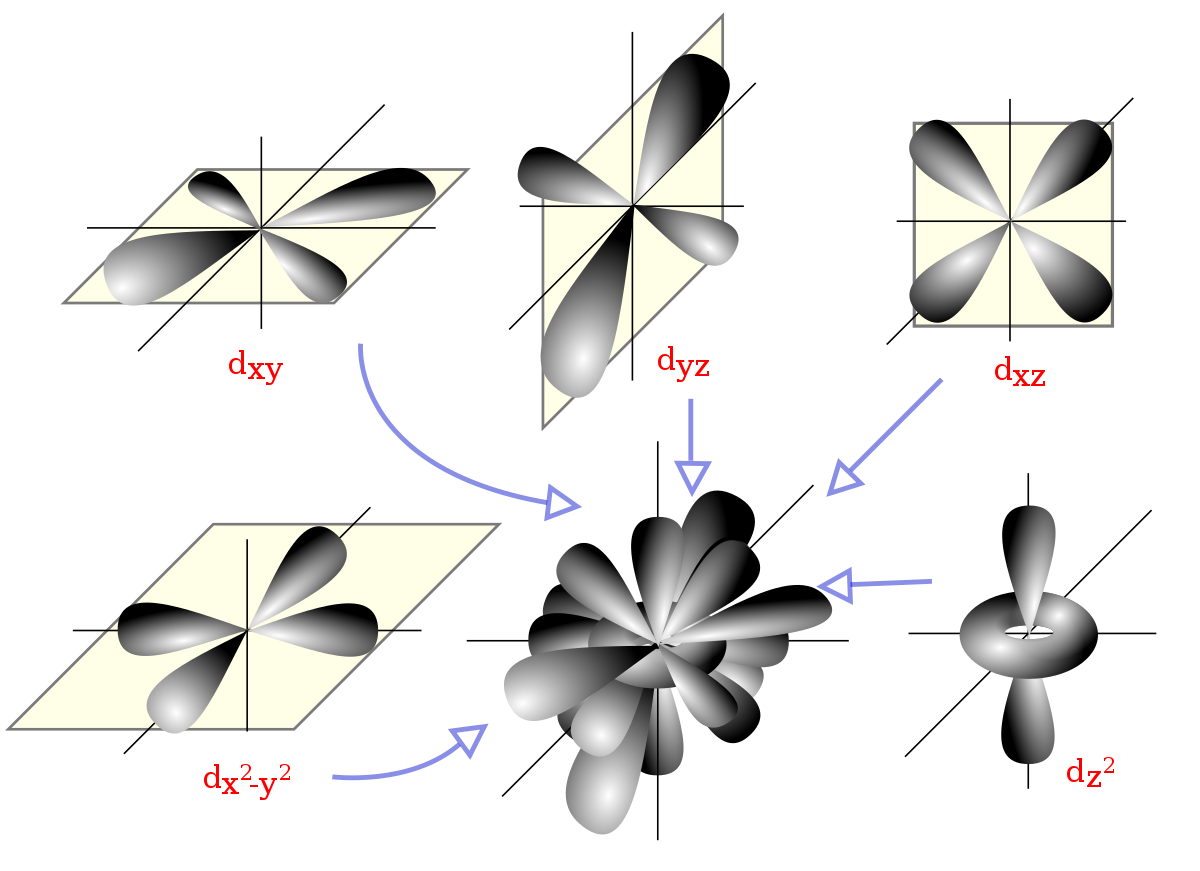
File D Orbitals Svg Wikimedia Commons

Ks9026 Set Of Five D Orbital Models Klinger Educational Products

D Orbital Shape Ewt

What Is The Shape Of A D Orbital Quora
A Energy Levels
Q Tbn 3aand9gcqulzhcplpqcomqengxsfh8zlrg Jw3 8b 3am6v0f U Tzuel Usqp Cau
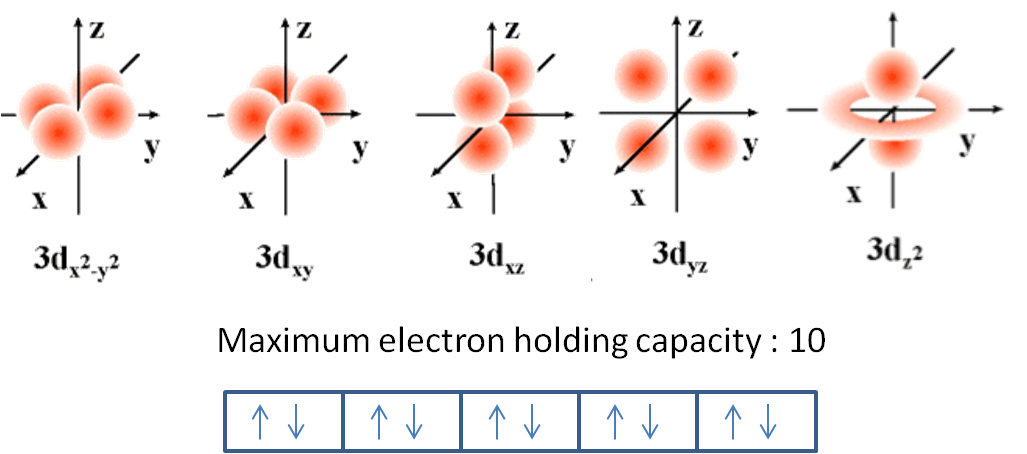
The Maximum Number Of Electrons Allowed In An Individual D Orbital Is Socratic

Shapes Of Orbitlas And Electronic Configuration Class 11 Chemistry

Colour Of Transition Metals
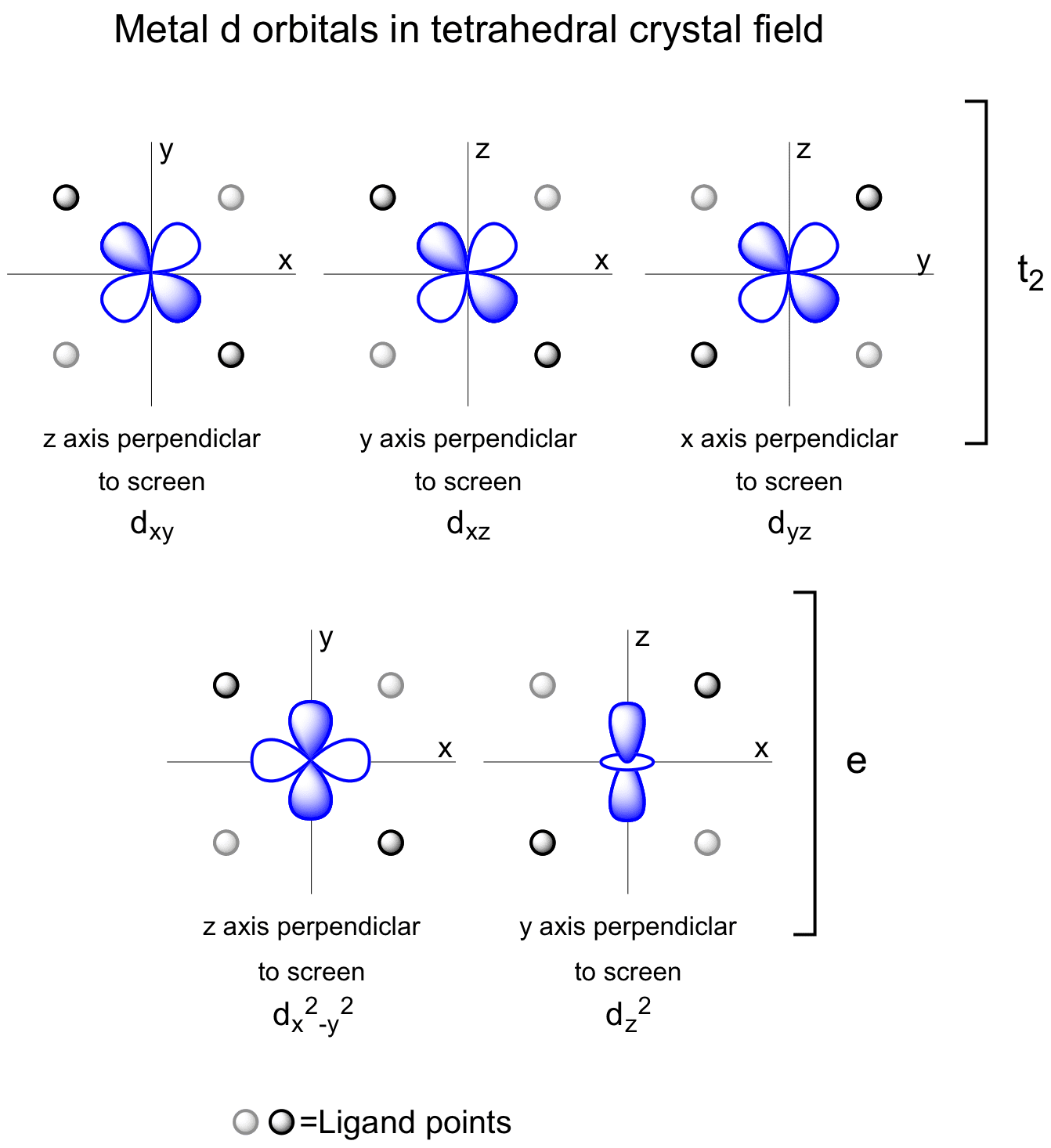
Metal D Orbitals In A Tetrahedral Crystal Field
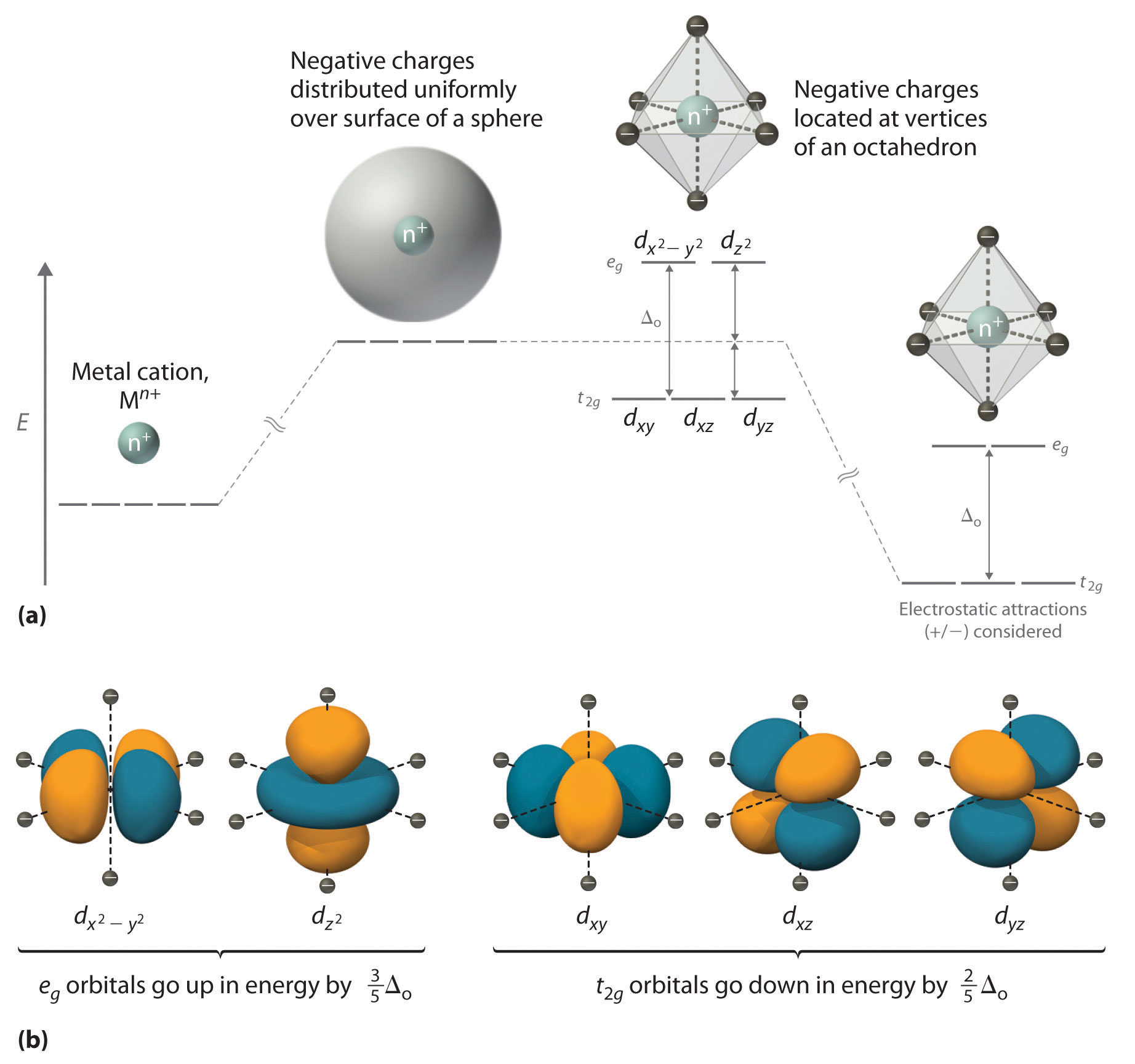
24 5 Bonding In Complex Ions Crystal Field Theory Chemistry Libretexts
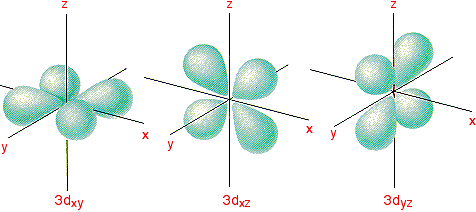
Complex Ions More About D Orbitals
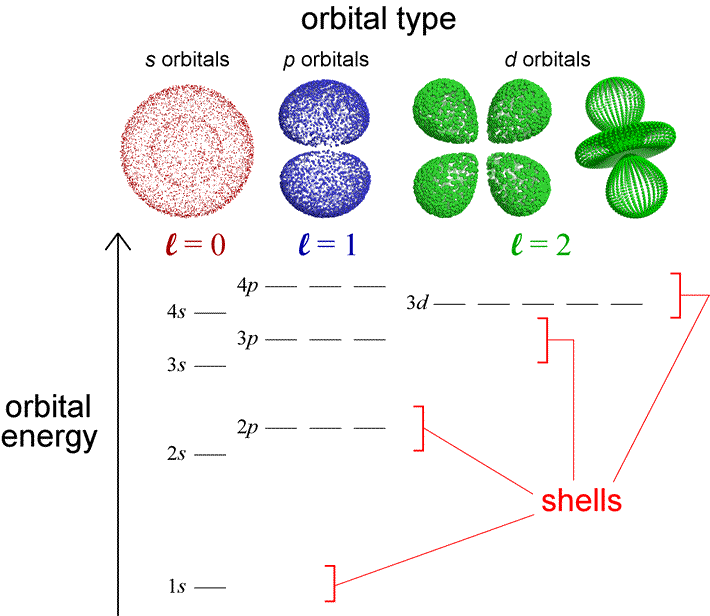
Chem 101 Lecture 5

Shapes Of Orbitals S P D Shapes

Quia Chem 4 2 The Quantum Model Of The Atom Bingo Version
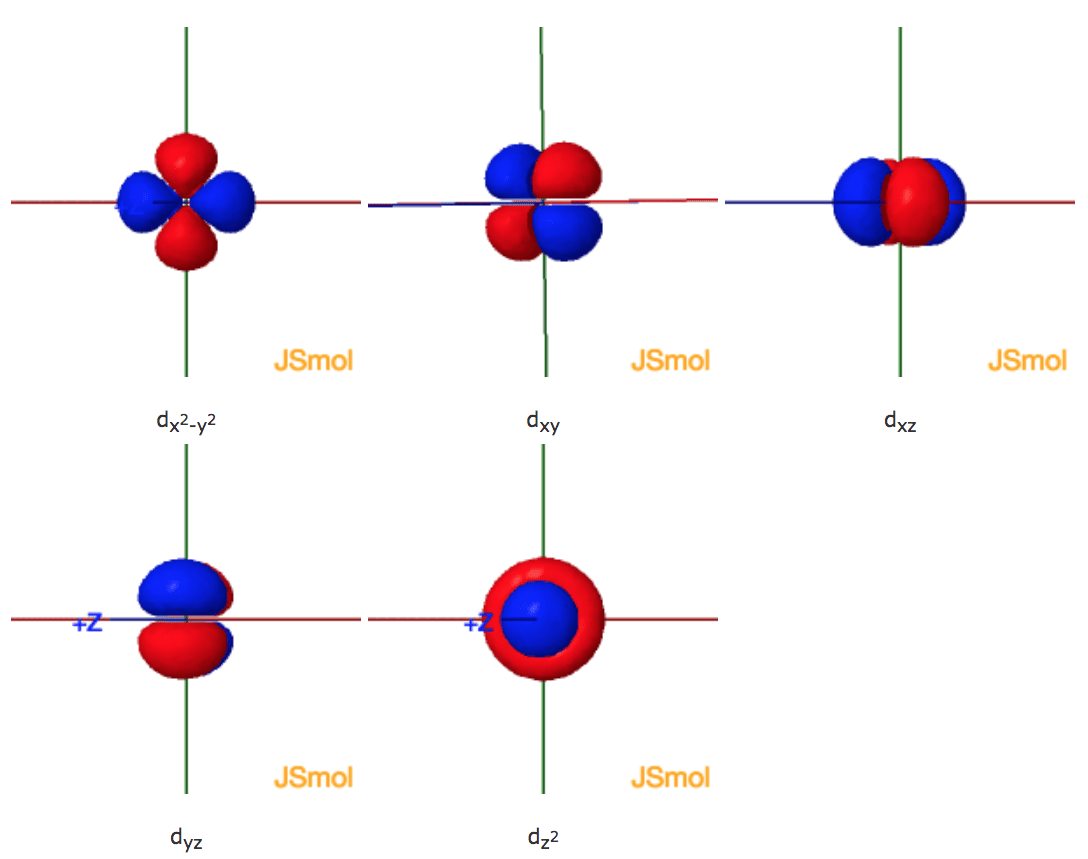
Shapes Of The 3d Orbitals In 3d
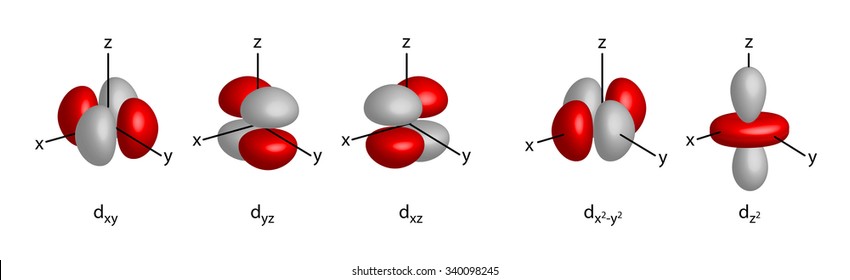
D Orbital Images Stock Photos Vectors Shutterstock

D Orbital Frustration Induced Ferromagnetic Monolayer Cu3o2 Sciencedirect

The 4 Types Of Orbitals Kensley

Crystal Field Theory Cft Sign Of Truth

1 The Five Different D Atomic Orbitals The Figure Is Adapted From Download Scientific Diagram
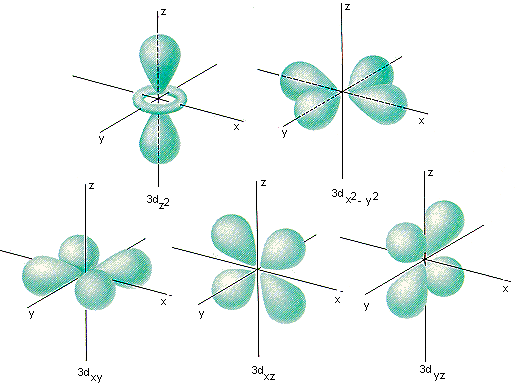
Quantum Numbers

What Is Symmetry Of P And D Orbitals In D 3h Chemistry Stack Exchange
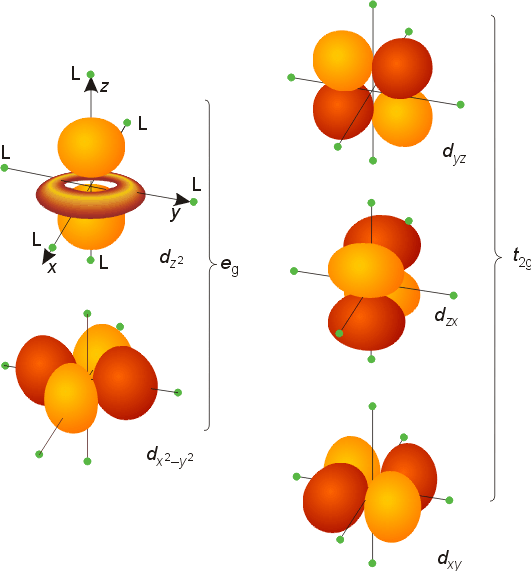
D Metal Complexes
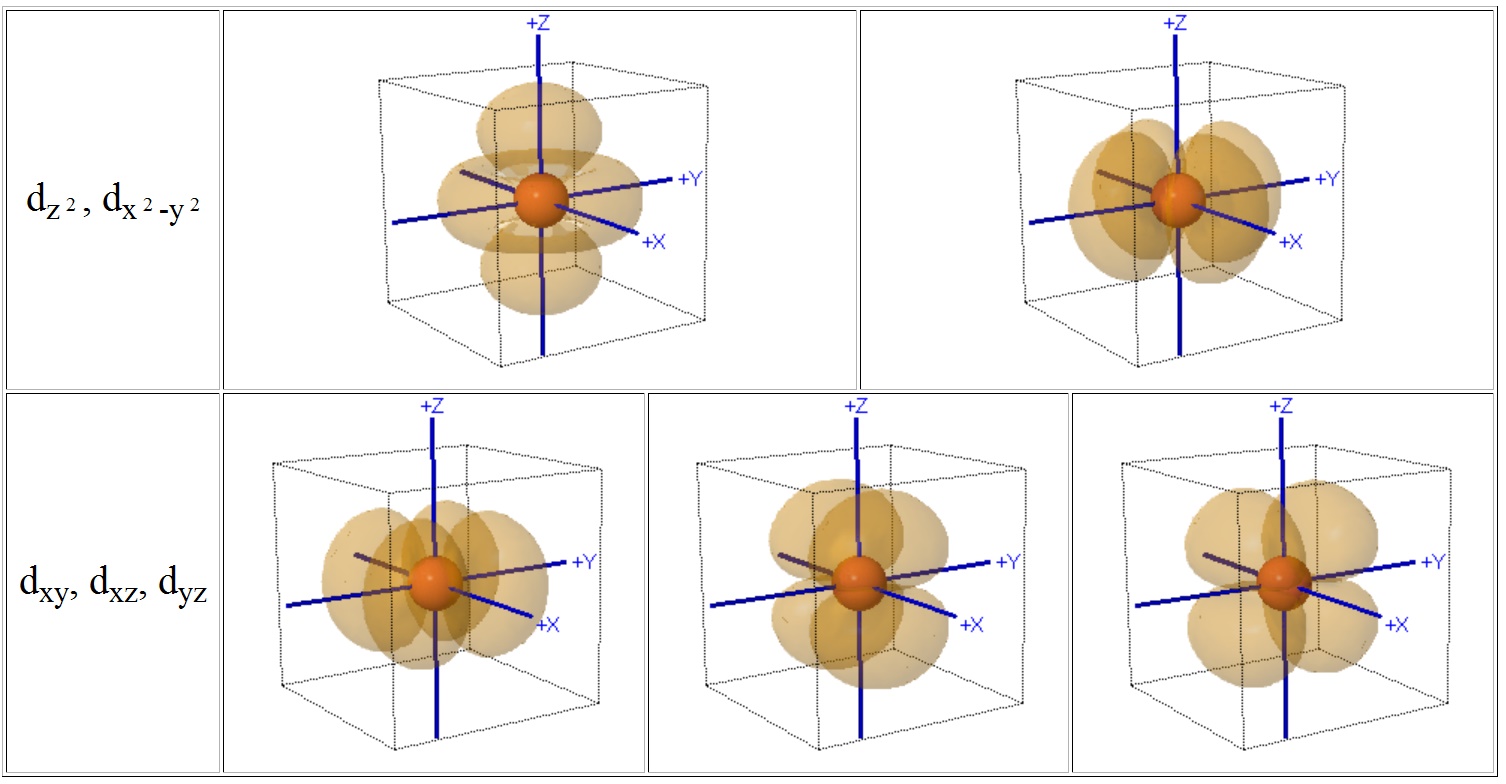
Crystal Field Theory Cft
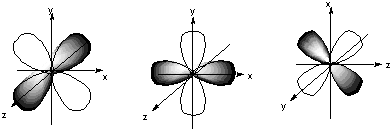
Chem 32 Virtual Manual



