Pcl3 Lewis Structure Molecular Geometry
What Is The Electron Dot Structure For Pcl 3 Socratic

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

Ch 6 5 Molecular Geometry Ppt Video Online Download

Ccl2o Lewis Structure How To Draw The Lewis Structure For Ccl2o Youtube

Solved 1 What Are The Factors That Effect Of The Molecul Chegg Com
Q Tbn 3aand9gcqvpukbsq6mp0xaykpnflqjzcnbay8e3m Xyb Anbbrfeafdd1w Usqp Cau
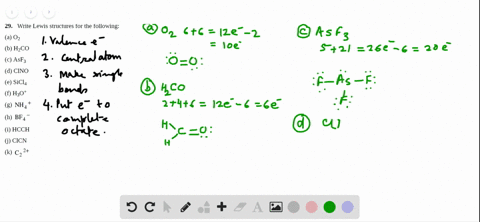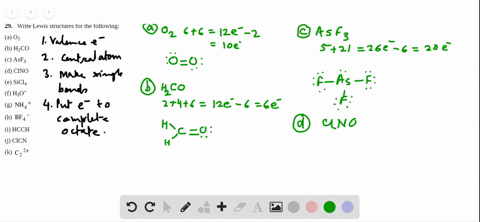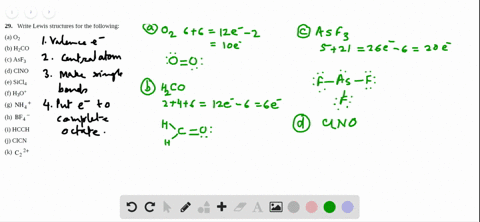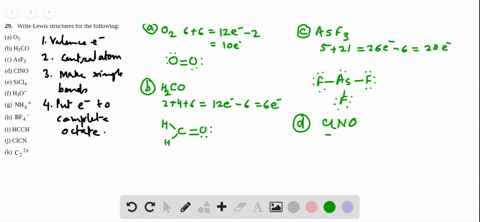
3 x Cl = 3 x 7 = 21.
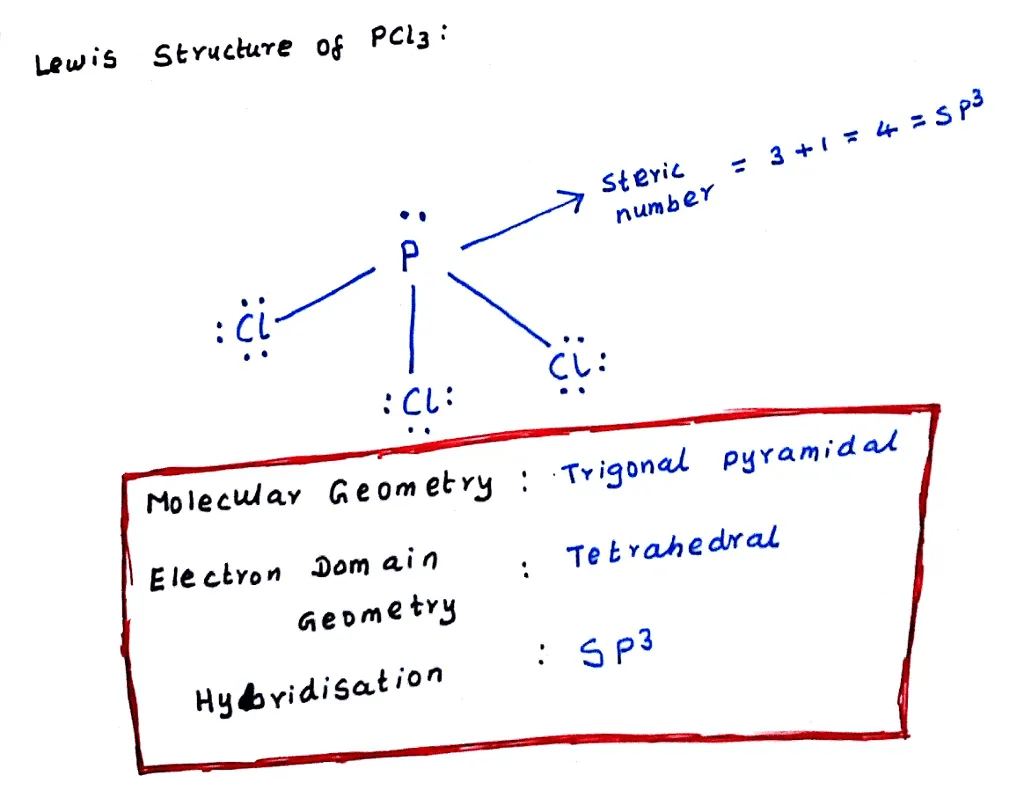
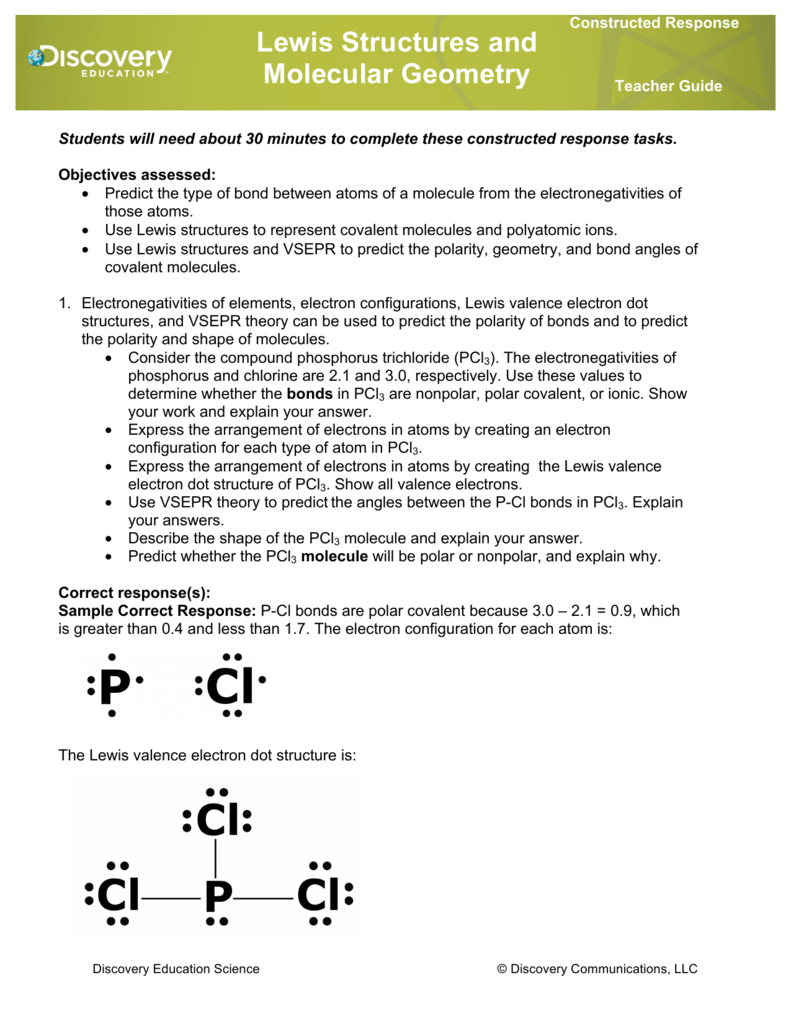
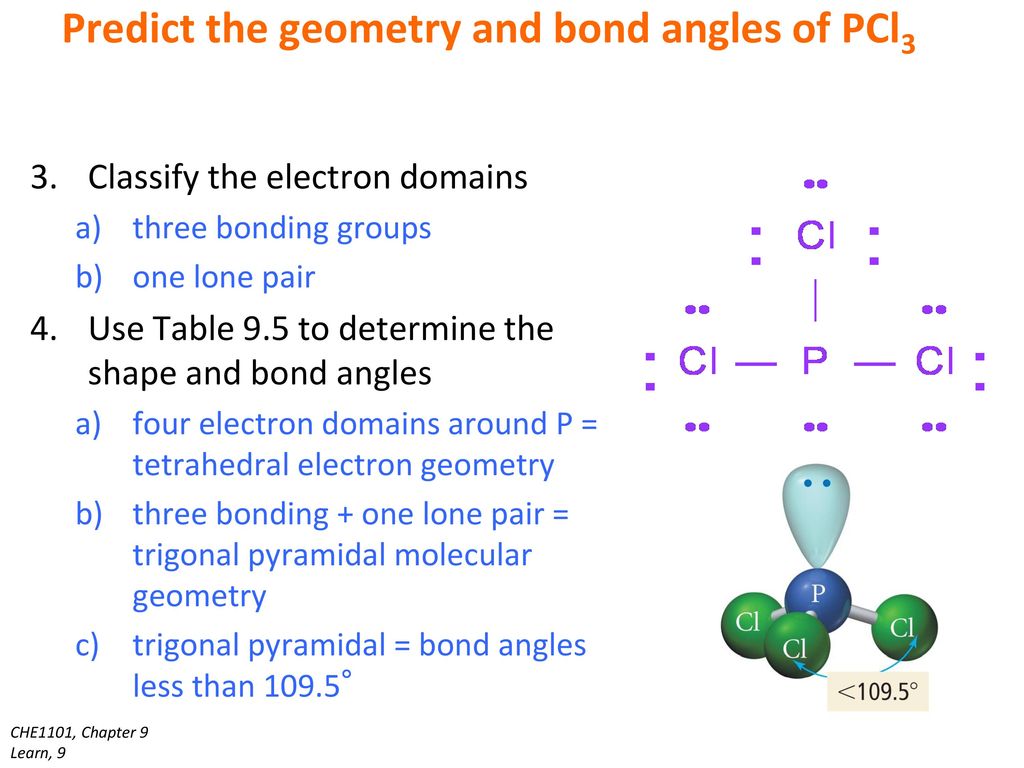
Pcl3 lewis structure molecular geometry. For the PCl3 Lewis structure we first count the valence electron. K is a plus one cation and ClO4 is a minus one anion. Since SiO2 has two places of electron concentration and no lone pairs, it is linear.
The electron arrangement and molecular shape are both linear. You can determine its molecular geometry by drawing its Lewis structure. Ball and stick model of pcl3 course hero.
It is a toxic and volatile liquid which reacts violently with water to release HCl gas. 3 e − Total VSE:. C2h4 Lewis Structure Molecular Geometry.
O2 Lewis Structure Molecular Geometry. Pcl3 ball and. A quick explanation of the molecular geometry of PCl3 including a description of the PCl3 bond angles.
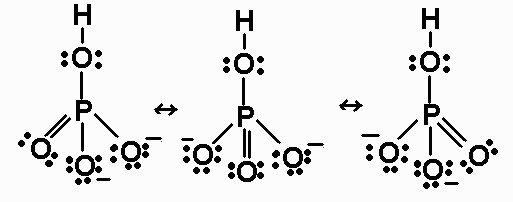
Trigonal pyramidal (based on tetrahedral) Note. In this example, we can draw two Lewis structures that are energetically equivalent to each other — that is, they have the same types of bonds, and the same types of formal charges on all of the structures.Both structures (2 and 3) must be used to represent the molecule’s structure.The actual molecule is an average of structures 2 and 3, which are called resonance structures. First draw the Lewis dot structure:.
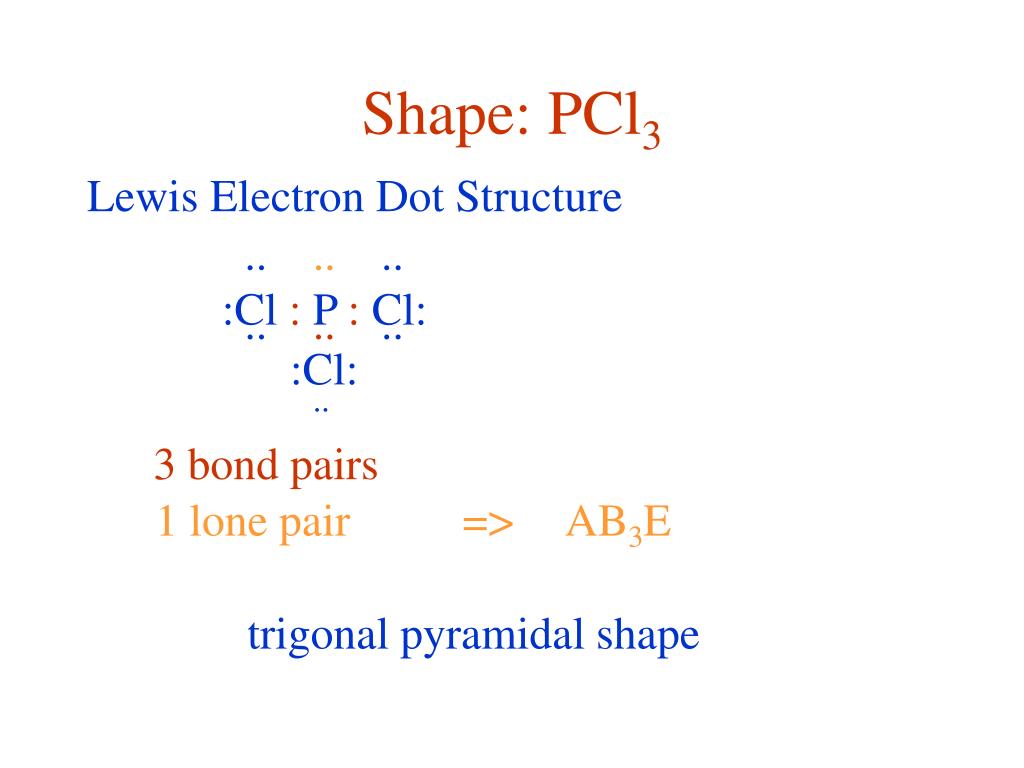
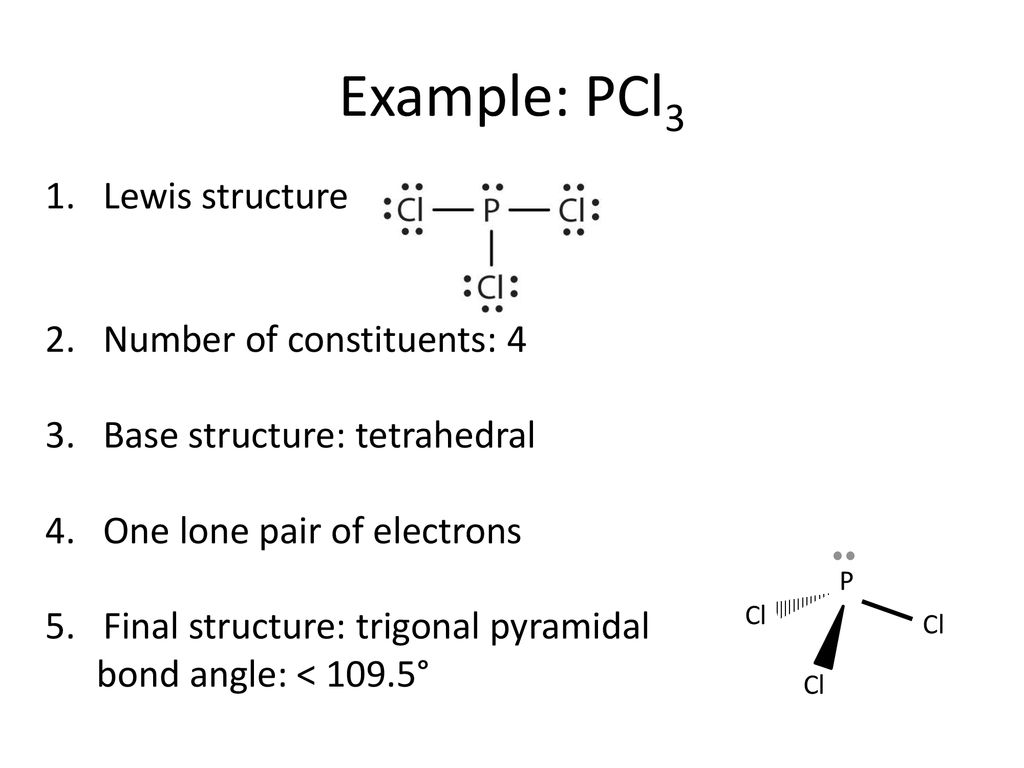
The molecular geometry of PCL3 is trigonal pyramidal with the partial charge distribution on the phosphorus. Janice Powell August 17, 18. They simply show the number and types of bonds.
C 2 H 2. The Lewis structure of PCl 3 is:. Structure is same as that of PCl5 i.e.
The central atom has at least one lone pair Identify the electron pair geometry of ClF₃. The central atom in SCl2 is surrounded by Answers:. To predict the Cl–P–Cl bond angles, look at the electron pairs around the central atom (here, P) and classify them as n (lone pair, here 1), σ (sigma, here, 3) and π (pi, here, 0).
PF 5 (phosphorus pentafluoride, a catalyst used in certain organic reactions) H 3 O + (hydronium ion) Given:. We can draw the Lewis structure on a sheet of paper. 1) what is the lewis structure for BrCl3?.
See full answer below. Boron trifluoride is the inorganic. The molecular geometry provides a good approximate of the polarity.
3) How Many Lone Pairs?. Predicting Electron-pair Geometry and Molecular Geometry:. Cl–P–Cl bond angle is less than 109° due to lone-pair:bonding-pair repulsion being greater than bonding-pair:bonding-pair repulsion;.
More Practice with Geometry of Lewis Structure. The most convenient way is. PCl3 - Phosphorus Trichloride:.
Bond, molecular geometry, Lewis structure, linear, trigonal planar, bent, tetrahedral, trigonal pyramidal:. Next, start with the central element P, draw its symbol and draw three lines from it connecting it to three chlorine symbols. If you are willing to understand the molecular structure of a compound, you can decide its polarity, reactivity, hybridization, shade, magnetism, and genetic movement.
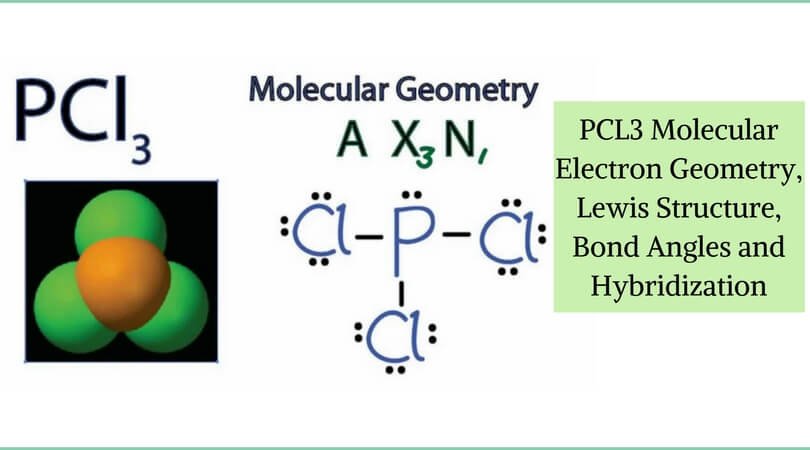
The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. The Lewis structure of PCl 3 is:. For bent molecular geometry when the electron-pair geometry is tetrahedral the bond angle is around 105 degrees.
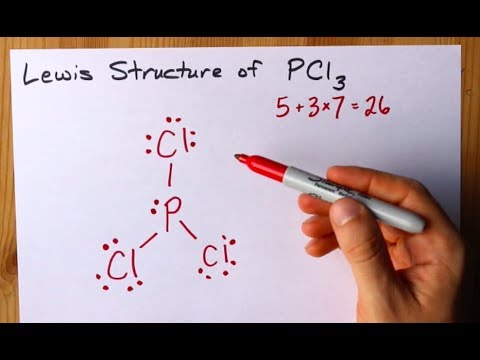
0 46,356 2 minutes read. Lewis dot structure for a PCl3 molecule:. If you mean how to work out the Lewis structure for PCl3, first count all available valence electrons as follows:.
BF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. The tellurium atom will the the central atom of the molecule, the four chlorine atoms being bonded to it. Generally, the Lewis structure is helpful to understand the molecular geometry of any given chemical compound.
The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. For each of the molecules or ions in this exercise,. By drawing the Lewis Structure, you will see that the most stable structure is O=Si=O.
This problem has been solved!. Sketch a ball and stick diagram of a water molecule. NO3^- == the Lewis structure will have two.
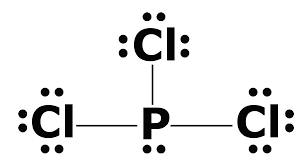
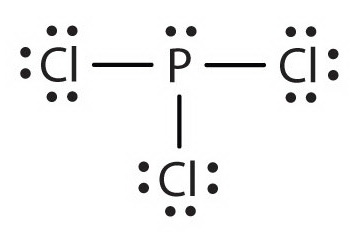
In the Lewis structure for PCl 3 there are a total of 26 valence electrons. A) NH3 b) ICl3 c) CO32-d) SO32-e) PCl3. 1) What Is The Lewis Structure For BrCl3?.
One way to answer these questions about molecular structure is to use a simple approach that builds on the Lewis-Dot structure approach called the Valence Shell Electron Pair Repulsion Model, or VSEPR model. The Organic Chemistry Tutor 18,348 views. Which of the following species has a Lewis structure with a molecular geometry similar to SO3?.
Name ER VE SP Hybridization Molecular Gerometry PCl3 Lewis Structure ER VE SP Hybridization Molecular Geometry Help Marvin JS Edit Drawing Sp Sp2 Sp3 CO2 Lewis Structure ER VE SP Hybridization Molecular Geometry Help Marvin JS Edit Drawing Sp Sp2 Sp3 SO3 Lewis Structure ER VE SP Hybridization Molecular Geometry Help Marvin JS Edit Drawing Sp Sp2 Sp3. But anyway, to get the shape of PCl3, you first have to draw the lewis structure. Species Lewis Dot Structure Molecular Geometry NO2+ NO2-NO3-PCl3.
Looking at the PCl3 Lewis structure we can see that th. But as Xenon does not form bonds easily, this compound is an exceptional case. K 2 HPO 4.
2) What Is The Electron Pair Geometry?. Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having the chemical formula PCl 3. Compound Lewis Structure Electronic Geometry Molecular Geometry.
Here is the Lewis structure of PCl3:. For trigonal pyramidal geometry the bond angle is slightly less than 109.5 degrees, around 107 degrees. Are you taking a class with me?.
Based on the molecular geometry, we can determine if the molecule is polar or nonpolar. Such compounds are known as ‘inorganic compounds’ as they are not the organic ones because of lacking Carbon. Hree pairs will be used in the chemical bonds between the P and Cl.
{eq}\boxed{all\:are\:polar\:molecules}{/eq} The figure below shows the Lewis structure and molecular geometry of the substances. Molecular Geometry which is also known as Molecular Structure is the three-dimensional construction or organization of particles in a molecule. Geometry of molecules is the place where you will find all the information about different chemical compound’s polarity, molecular geometry, lewis structure, etc.
Then draw the 3D molecular structure using VSEPR rules:. Lone Pairs on the Central Atom Predict the electron-pair geometry and molecular geometry of a water molecule. 4) What Is The Molecular Geometry?.
Lewis Dot Diagram Structure For PCl3, Molecular Geometry, Bond Angle, Hybridization, Polar or Nonpol - Duration:. Draw the Lewis structure for the molecular structure. The Lewis structure of phosphorus trichloride is given below:.
This step by step chemistry video will show you how to draw Lewis structures and determine molecular geometry using phosphorus pentachloride (PCl5) as an exa. Which of the following species has a Lewis structure with a molecular geometry similar to SO3?. How do you find the number of valence electrons and how do you know where to place those valence electrons as bonds or lone pairs?What about for a species with a cation and anion like KClO4?.
The phosphorus has an electronegativity value of 2.19 and chlorine comes with 3.16. C 2 H 4. A) two single bonds and no lone pairs of electrons.
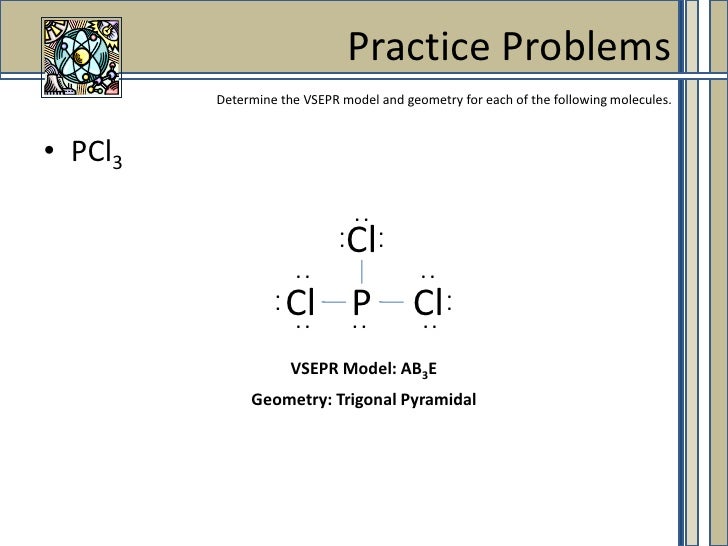
Because of larger size of Br atom, and in order to minimize the bond pair-. It has a trigonal pyramidal shape, owing to the lone pairs on the phosphorus. Therefore, the electron pair geometry is tetrahedral and the molecular geometry is trigonal pyramidal.
That will tell you both the hybridization and the bond angles. PCl 3 is similar to PBr 3 and PF 3.If you can do those Lewis structures PCl 5 will be easy.;. 3) how many lone pairs?.
The molecular geometry of Xenon Difluoride can be understood by knowing the VSEPR theory. Drawing the Lewis Structure for PCl 3. 5 + 21 = 26 available valence electrons.
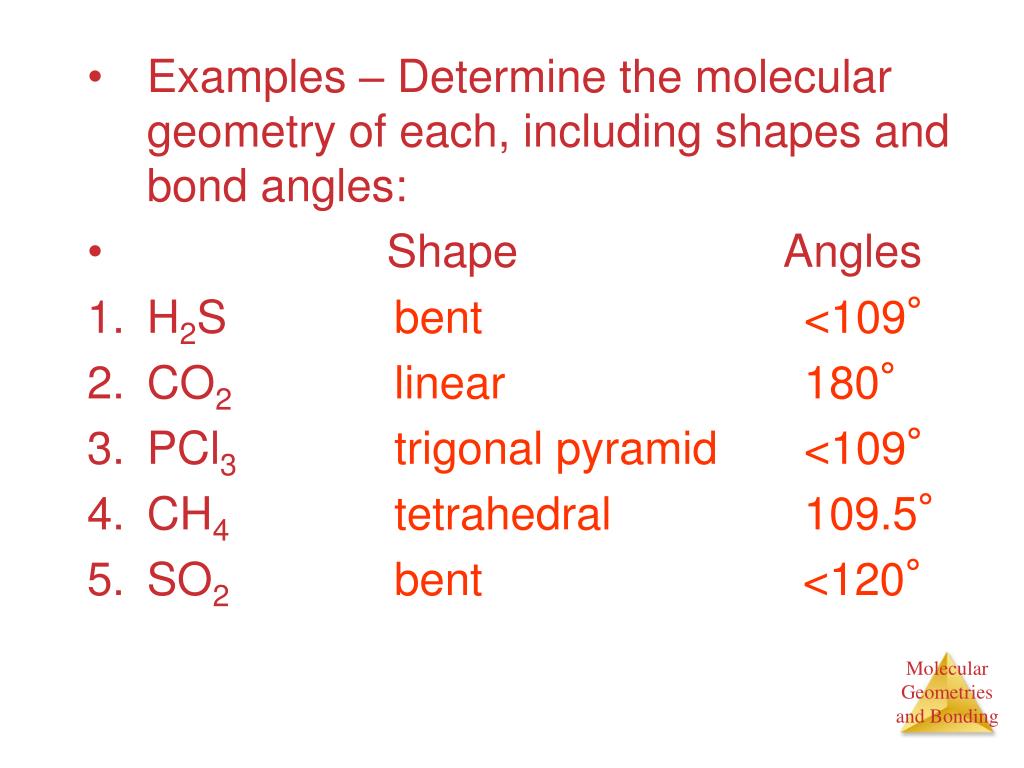
The molecular structure of a molecule will differ from its electron-pair geometry if:. The molecular geometry of PCl 3 is trigonal pyramidal. Solution The Lewis structure of H 2 O indicates that there are four regions of high electron density around the oxygen atom:.
Media portfolio pearson education. With five valence electrons from the phosphorus atom and seven valence electrons from each of three chlorine atoms, 26 electrons are available to satisfy octets. But we describe molecular geometry on the basis of the geometry of the ATOMS, not on the electron pairs.
In the PCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center.;. Lets consider the Lewis structure for CCl 4. How do you write the lewis structure for PCl3?.
5) Lewis structures, however, do not indicate the shapes of molecules;. 1 lone pair and 3 single bonds. Only interesting thing about PCl3Br2 is which atom Cl orBr should be placed at Axial or equitorial bonds.
5 e − 3 x Cl contibute:. Two lone pairs and two chemical bonds:. Does the Cl use an expanded octet to form bonds with the oxygen atoms?.
To a first approximation, these electron pairs arrange themselves in a tetrahedronwith ideal bond angles of 109.5^@. Phosphorus Trichloride, PCl3 Molecular Geometry & Polarity. A level chemistry ocr salters molecular geometry.
Chemistry drawings how to draw chemistry structures. A step-by-step explanation of how to draw the PCl3 Lewis Structure (Phosphorus Trichloride). The structure shows that there are 4 electron groups around P i.e.
"Trigonal pyramidal" We gots PCl_3, and around the central phosphorus atom there are 3xx"bonding electron pairs", the P-Cl bonds, and a "phosphorus-centred" lone pair. 4) what is the molecular geometry?. The molecule has a total of 34 valence electrons, 6 from the tellurium atom and 7 from each of the four chlorine atoms.
Draw the Lewis electron structure of the molecule or polyatomic ion. B) two single bonds and one lone pair of electrons. You can predict the bond angles of tellurium tetrachloride by looking at its molecular geometry.
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Learn how to draw a Lewis Structure and how to use it to assign a molecule's shape (geometry). Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons.
In the Lewis Structure Drawing Tool, change the molecule as needed for an incorrect answer. Using the VSEPR model, predict the molecular geometry of each molecule or ion. Drawing the lewis structure for clo 2 university of maryland.
1 x P = 1 x 5 = 5. Click and drag the molecle to rotate it. It is the well-known fact that if there is a vast difference of the electronegativity, there are more chances of polarity.
To do that, you have to get the total number of valence electrons (electrons in the atom's most outer electron shell) P will have 5 electrons in its valence shell and Cl will have 7 electrons in its valence shell. PCl3 == three bonding pairs and one lone pair. You may have heard about the chemical compound that lacks C-H bonds.
If so, feel free to rate me at. Take the sum of n+σ (here, 4). The idea behind this approach is that the structure around a given atom is determined principally by minimizing electron repulsions.

Molecular Geometry And Bonding Theories Ppt Download
Q Tbn 3aand9gcsiivegpjqtmklkikj94ec3v0doatidfg1xzw Usqp Cau

Pcl3 Phosphorus Trichloride Structure Molecular Mass Properties And Uses
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
Sf4 Molecular Geometry Lewis Structure And Polarity Explained
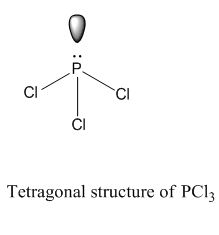
What Type Of Bond Is Pcl3

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube
Mastmedical Org Ourpages Auto 13 11 15 Vsepr and molecular geometry 12 nov Pdf
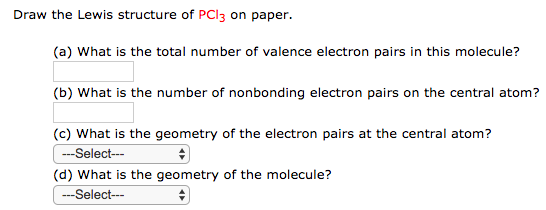
Solved Draw The Lewis Structure Of Pcl3 On Paper A Wha Chegg Com

Lewis Structures And Hybridizations For Common Molecules

Chapter10 Tro 4 Based On The Lewis Structure The Number Of Electron Domains In The Valence Shell Of The Co Molecule Is A 1 B 2 C 3 D 4 E 5 Pdf Free Download

Ph3 Molecular Geometry Shape And Bond Angles Note Actual Bond Angle Is 93 5 Degrees Youtube

Solved I Did My Work And I Felt Like I M Missing Somethin Chegg Com
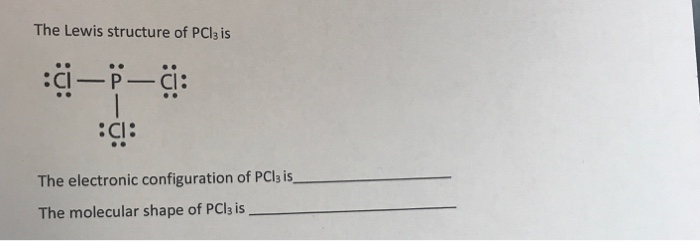
Solved The Lewis Structure Of Pcl3 Is P C C The Elec Chegg Com
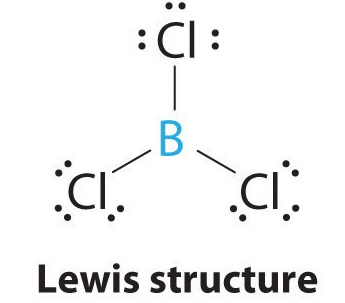
9 7 The Shapes Of Molecules Chemistry Libretexts

Solid Pcl3 Behaves As Ionic Compound Why Brainly In

Exceptions To The Octet Rule Chemistry Master
Untitled
Ppt Chapter 9 Molecular Geometries And Bonding Theories Powerpoint Presentation Id
Www Mctcteach Org Chemistry C10 C10 Handouts Molecular modeling v 8 18 Pdf
Chem Ntou Edu Tw Bin Downloadfile Php File Wvhsmflxtm9mekl5tdncmflwodjnvgd3whpzek9uuxhovgxmtkrnd09euxvjr1jt Fname Utjoagniumxjauf4tuy5b2jxehdar1l1y0dsbq
Explain The Non Linear Shape Of H2s And Non Planar Shape Of Pcl3 Using Valence Shell Electron Pair Repulsion Theory Studyrankersonline

Chclo Lewis Structure How To Draw The Lewis Structure For Chclo Youtube
Print Ch9 Study Packet Flashcards Easy Notecards

What Is The Molecular Geometry Of Pcl3 Study Com
Solved Hi Can Somebody Answer The Circled Questions Only Chegg Com

Pcl5 Lewis Structure

Use The Vsepr Method To Predict The Molecu Clutch Prep
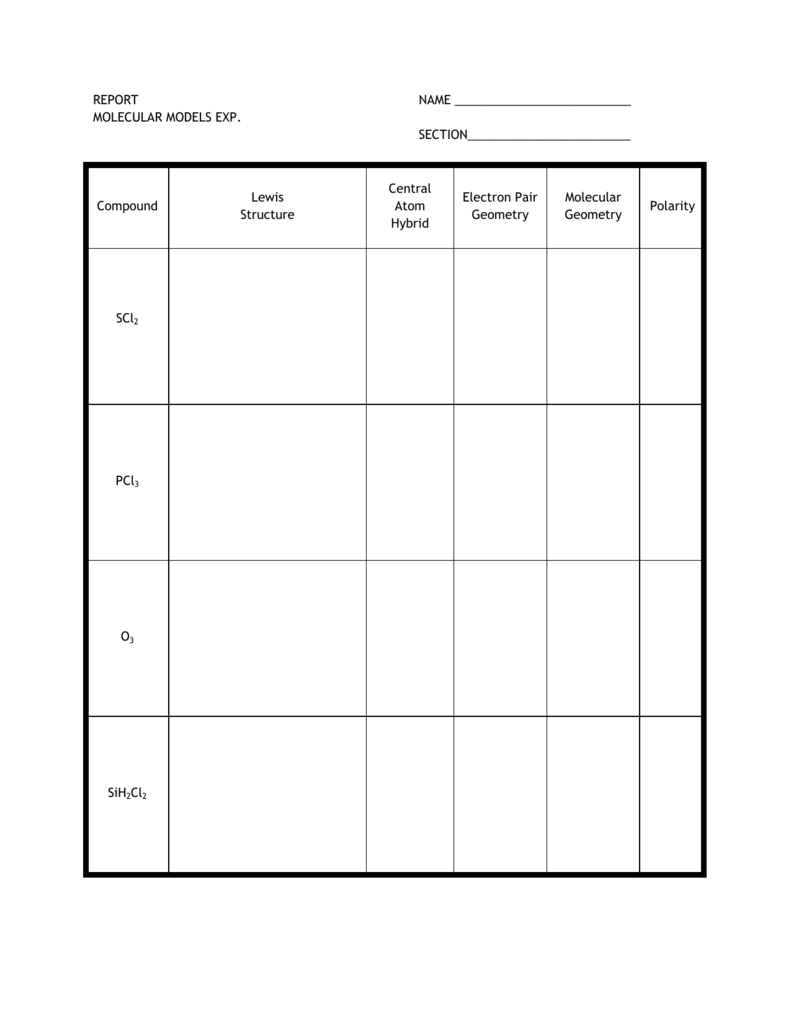
Report Name Molecular Models Exp Ars
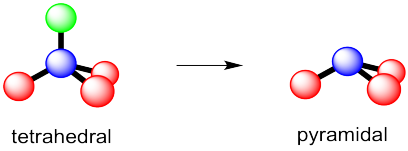
Question C9022 Socratic
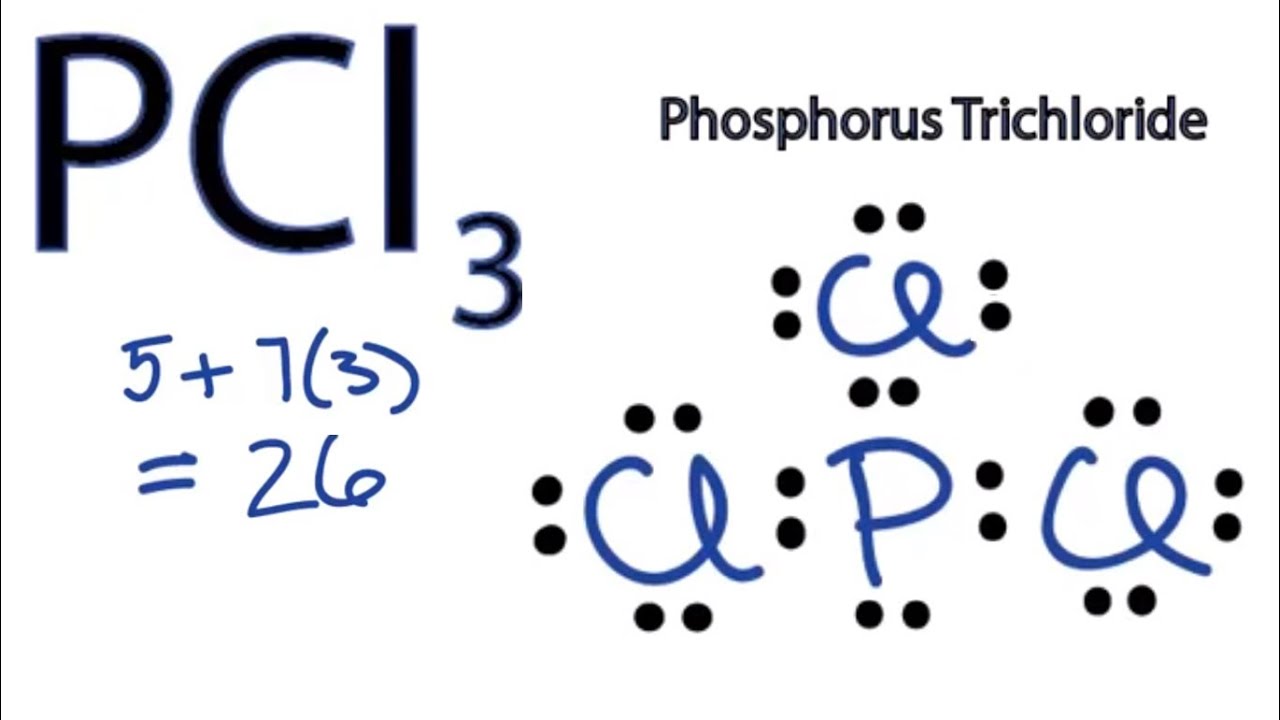
Pcl3 Lewis Structure How To Draw The Lewis Structure For Pcl3 Phosphorus Trichloride Youtube
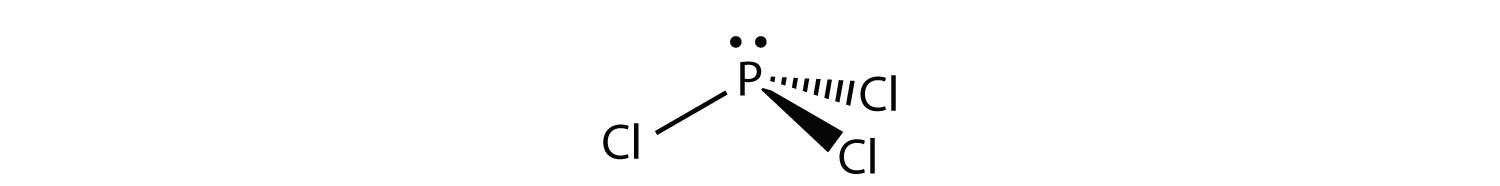
Molecular Shapes

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Explain The Structure Of Pcl3 And Pcl5 Chemistry Topperlearning Com C9yvmoxx
Junsay Files Wordpress Com 10 09 Experiment 19 Bonding Pdf

Chemistry A Molecular Approach 1st Edition Ppt Video Online Download
Junsay Files Wordpress Com 10 09 Experiment 19 Bonding Pdf

Pcl3 Lewis Structure

What Is The Molecular Shape Of Pcl3 Quora

Ppt Molecular Geometry And Chemical Bonding Theory Powerpoint Presentation Id

Problem Set 6

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

The Bond Angles Increase Steadily In The S Clutch Prep
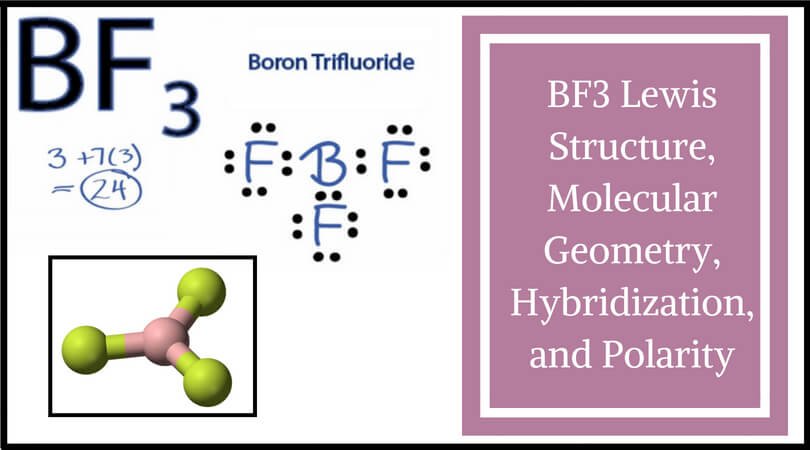
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

Molecular Geometry Ac
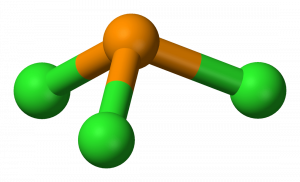
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
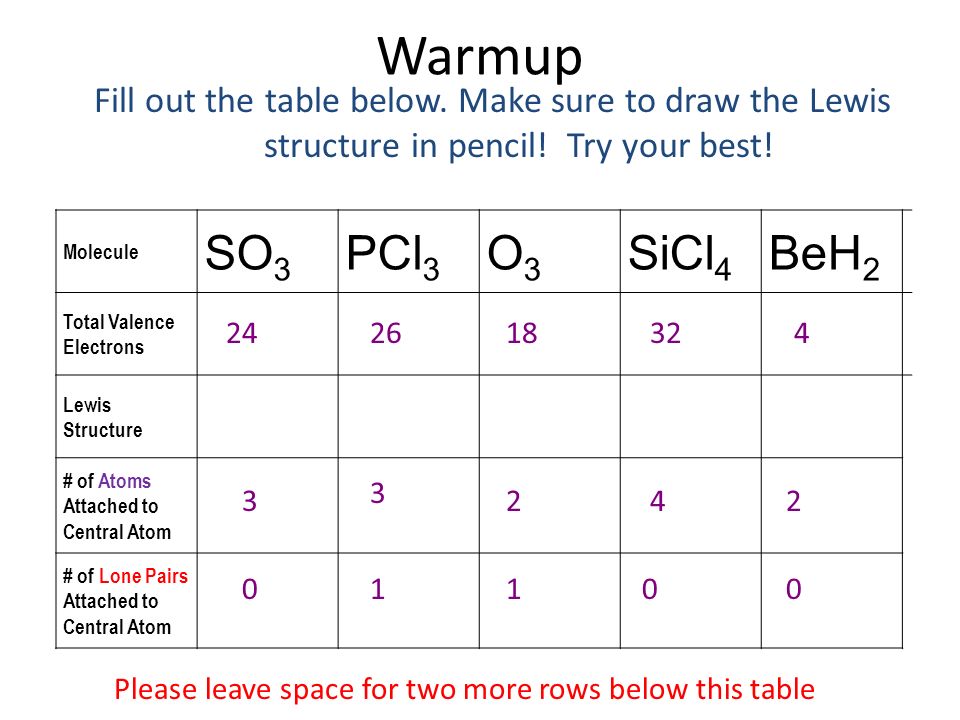
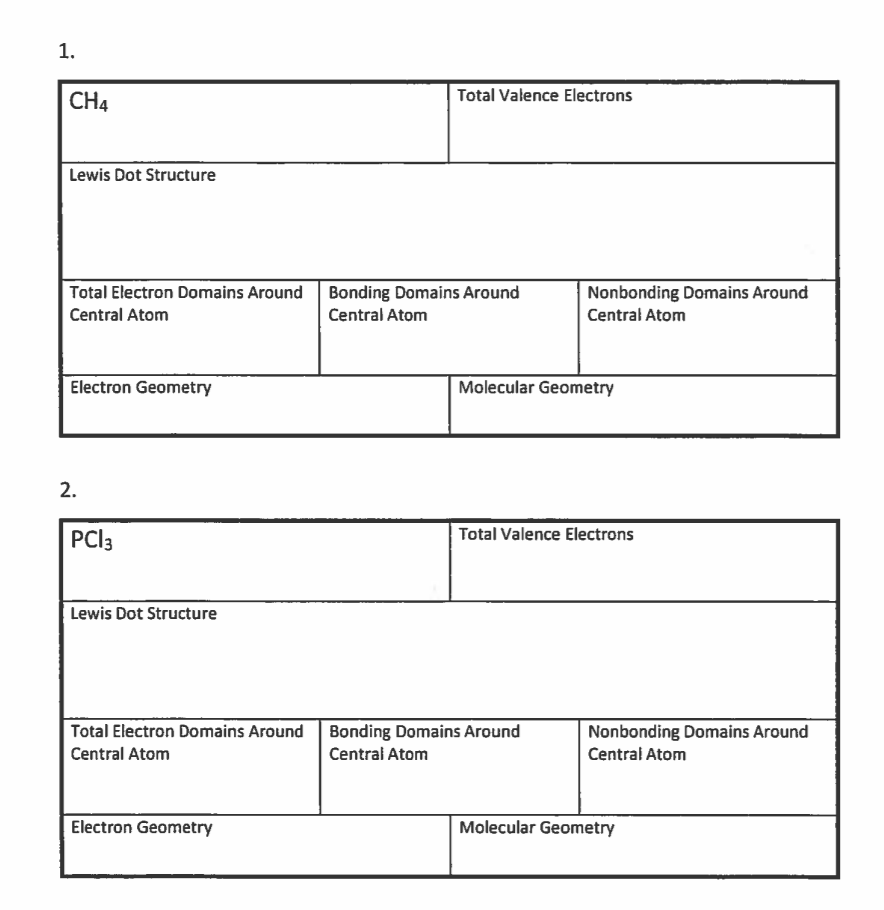
Warmup Fill Out The Table Below Make Sure To Draw The Lewis Structure In Pencil Try Your Best Molecule So3 Pcl3 O3 Sicl4 Beh2 Total Valence Electrons Ppt Video Online Download

Solved Pre Laboratory Assignment 1 Draw The Best Lewis S Chegg Com

I3 Lewis Structure Shape Hybridization And Polarity
1

Pcl3 Lewis Structure And Molecular Geometry Youtube
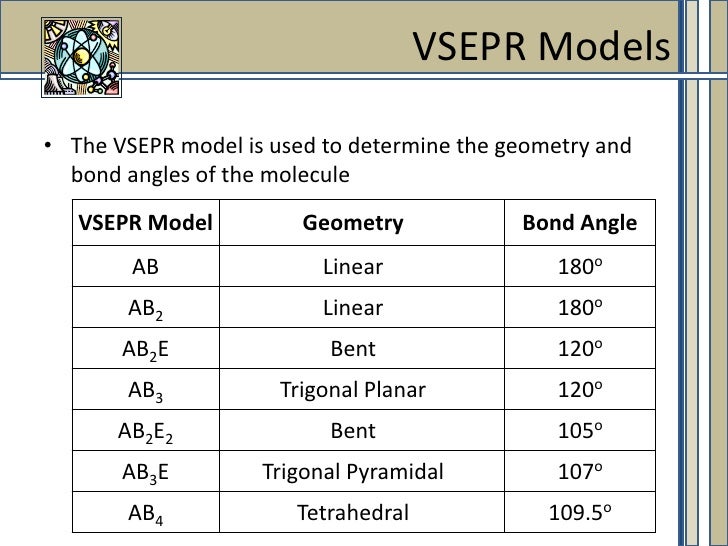
Molecular Geometry Ac
Mastmedical Org Ourpages Auto 13 11 15 Vsepr and molecular geometry 12 nov Pdf

The Bond Angles Increase Steadily In The S Clutch Prep

Which Of The Following Molecules Ions Has A Pyramidal Shape Clutch Prep

Chemistry 114 Chapter 10 Flashcards Quizlet

Predict The Molecular Geometry Of The Compound Pcl3 Using Vsepr Study Com
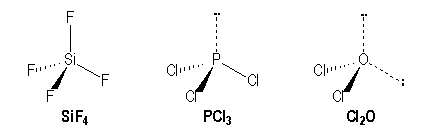
Vsepr Method By G Dupuis And N Berland

Occurrence Preparation And Properties Of Phosphorus Chemistry For Majors
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Chem Test 1 Flashcards Quizlet
Q Tbn 3aand9gcttrltvdnkotmhbe2mj2d52zhrv5vdfiwxavc9xjal I6svroes Usqp Cau

Diagram Nf3 Lewis Diagram Full Version Hd Quality Lewis Diagram Orquestralivre Arcieriarcobaleno It
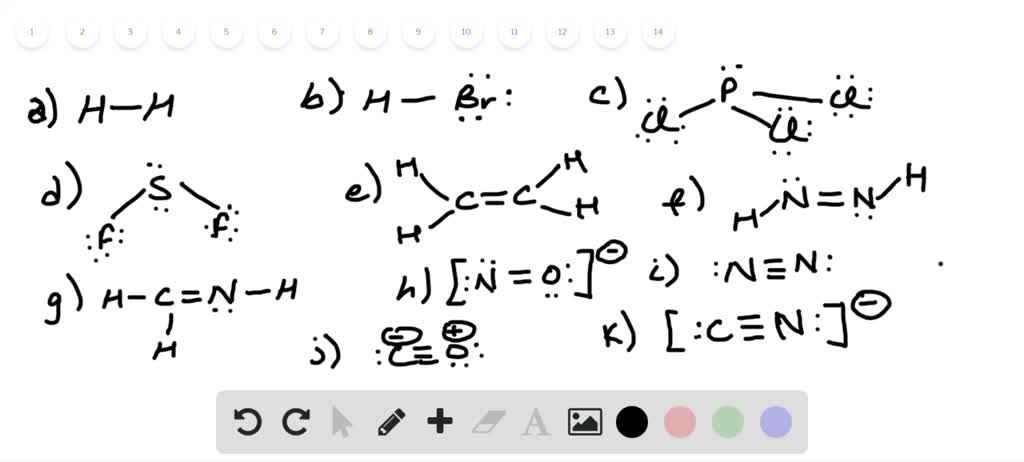
Write Lewis Structures For The Following A H2
What Is The Bond Angle Of Pcl3 Quora

Pcl3 Molecular Geometry
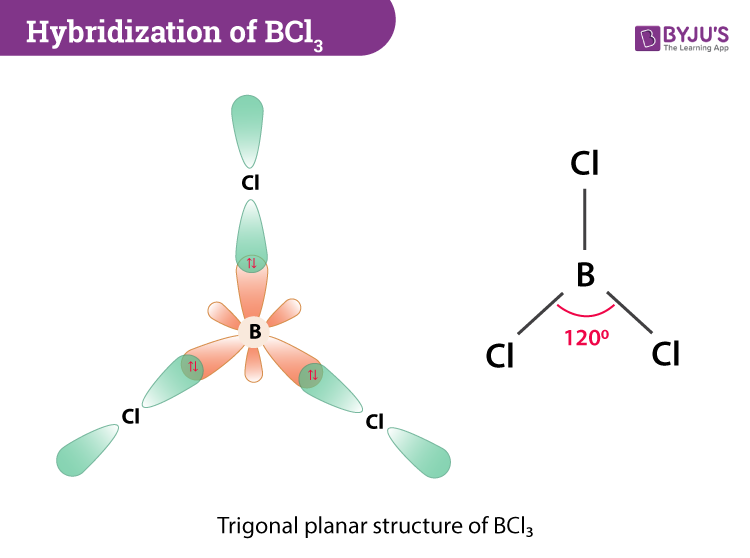
Hybridization Of l3 Hybridization Of Boron In l3

Pcl3 Molecular Geometry Shape And Bond Angles Youtube
Oneclass Write Lewis Structures For Pcl3

How Is The Electron Dot Structure Of Pcl3 Determined Quora
Solved Ch4 Total Valence Electrons Lewis Dot Structure To Chegg Com

Procedure For Predicting Molecular Geometries I I

Rank The Members Of The Set Of Compounds Pcl3 Pbr3 Pf3 In Order Of Decreasing Ionic Character Of Their Bonds Use Par Homeworklib
Lewis Structures And Molecular Geometry
Q Tbn 3aand9gcqd4mttlm Lxof4eicktwu61s1r1uqgmy1pgwcrao8cnbdkit O Usqp Cau
Molecular Geometry Ac
Question C9022 Socratic

Calculate Molecular Mass Of Pcl3 Brainly In
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles

Announcements Be Respectful No Electronics Please Ppt Download
12books Lardbucket Org Pdfs Principles Of General Chemistry V1 0 S13 Molecular Geometry And Covalen Pdf

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

How Many Lone Pair Of Electrons Are Present On The Central Atom Of Ch4 H2o Nh3 Pcl3 And Pcl5 Molecules
Http Www Nobraintoosmall Co Nz Students Chemistry Ncea Level2 Pdfs Che Shape0611 Plus Answers Pdf
Based On The Vsepr Theory What Is The Molecular Geometry Of A Molecule Of Pi3 Socratic

Sample Exercise 9 1 Using The Vsepr Model Ppt Video Online Download
Chem Ntou Edu Tw Bin Downloadfile Php File Wvhsmflxtm9mekl5tdncmflwodjnvgd3whpzek9uuxhovgxmtkrnd09euxvjr1jt Fname Utjoagniumxjauf4tuy5b2jxehdar1l1y0dsbq
Valenceshellelectronpairrepulsion Ppt Download

Pcl3 Lewis Structure And Molecular Geometry Youtube
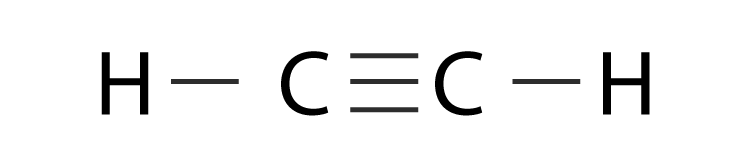
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne
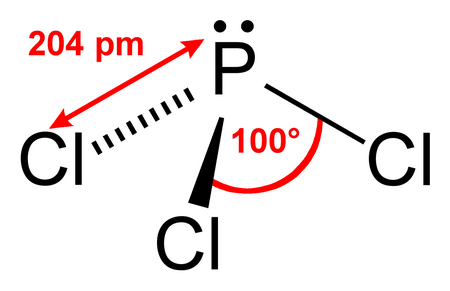
Question C9022 Socratic
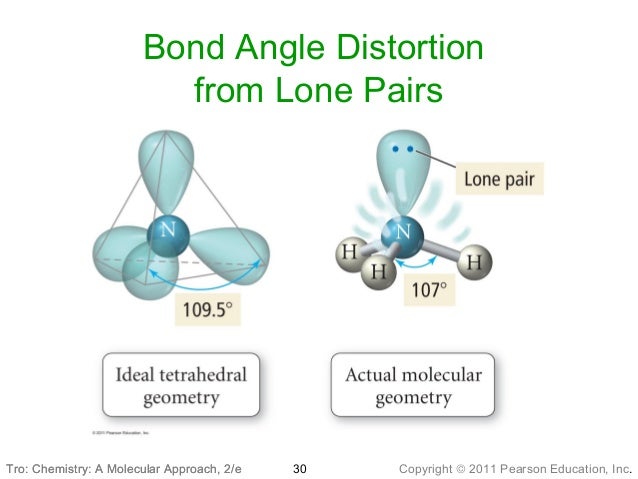
10 Lecture
Explain The Non Linear Shape Of H2s And Non Planar Shape Of Pcl3 Using Valence Shell Electron Pair Repulsion Theory Sarthaks Econnect Largest Online Education Community
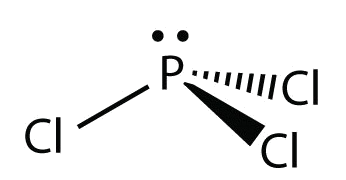
4 11 Molecular Shapes The Vsepr Theory Chemistry Libretexts

Chem Test 1 Flashcards Quizlet
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

Pcl3 Lewis Structure How To Draw The Lewis Structure For Pcl3 Phosphorus Trichloride Youtube
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube



