6s Orbital
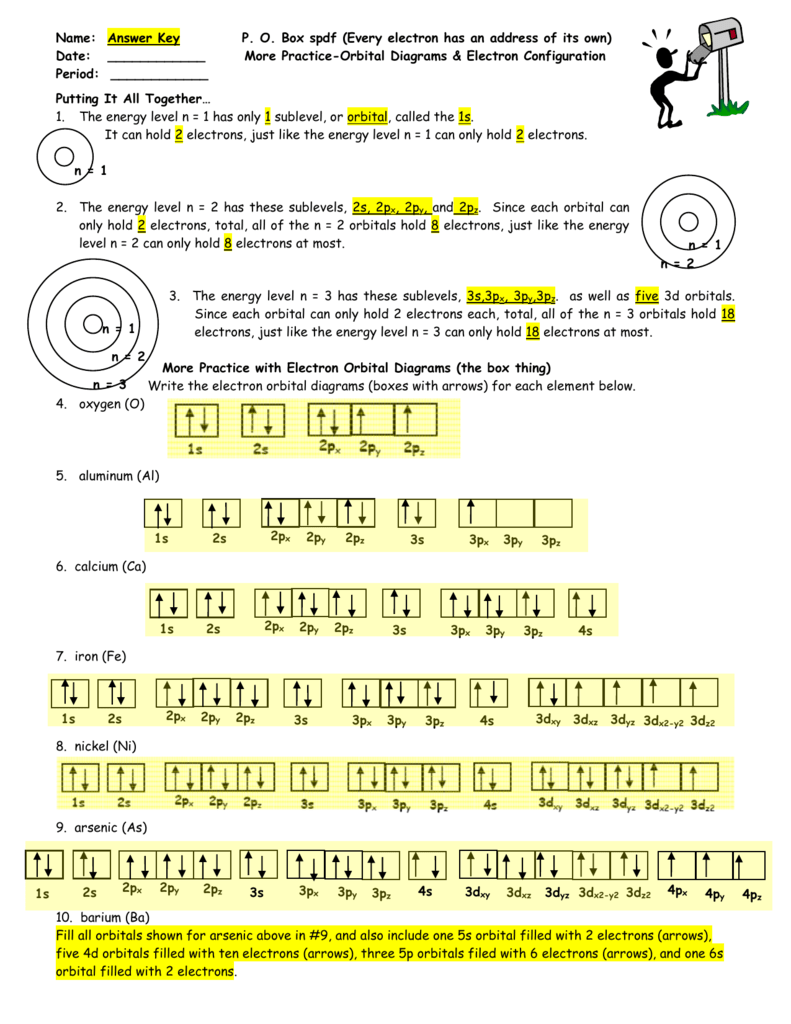
Po Box Spdf Worksheet Answer Key

Section 3 Electron Configurations Chapter 9 Electrons In Atoms And The Periodic Table Ppt Download

Energy Levels Of The S And D Orbitals In A Single Copper Silver And Download Scientific Diagram
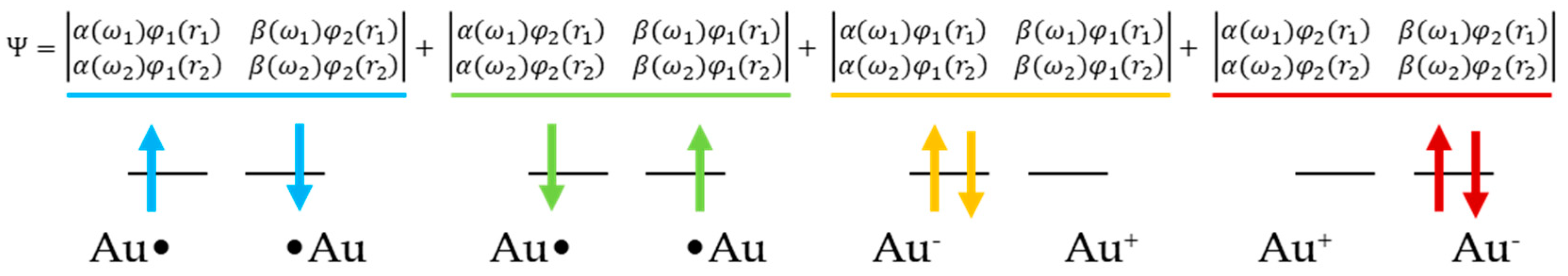
Molecules Free Full Text Extent Of Spin Contamination Errors In Dft Plane Wave Calculation Of Surfaces A Case Of Au Atom Aggregation On A Mgo Surface Html

Upper Radial Component Of The Cs 6s Orbitals Thick Solid Line Download Scientific Diagram
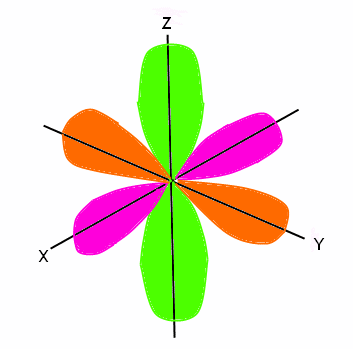
Building Up The Periodic Table
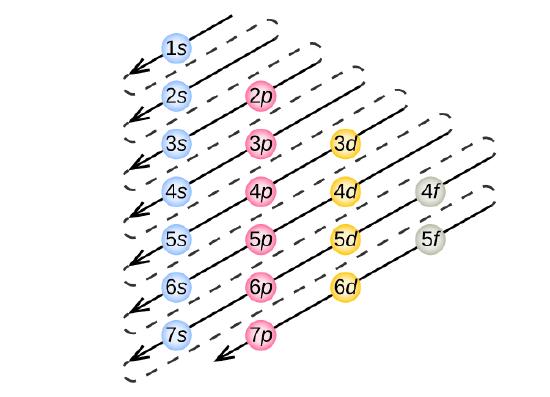
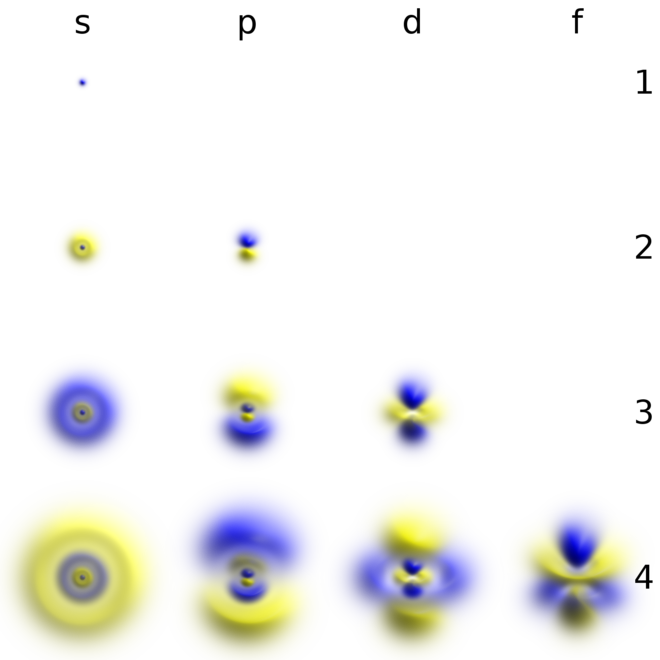
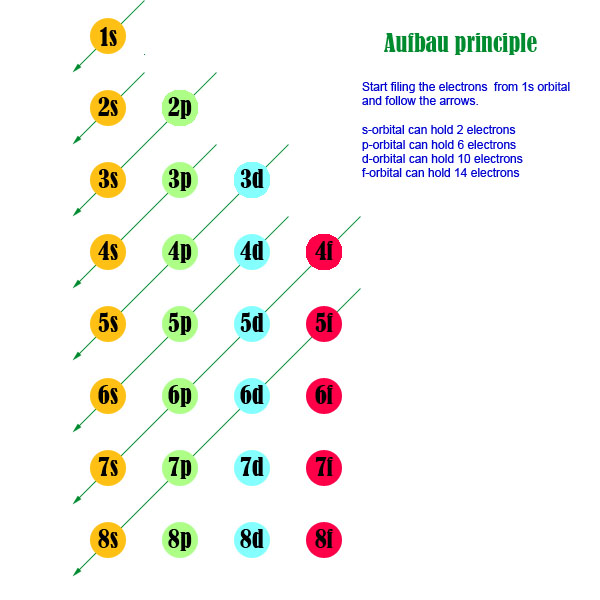
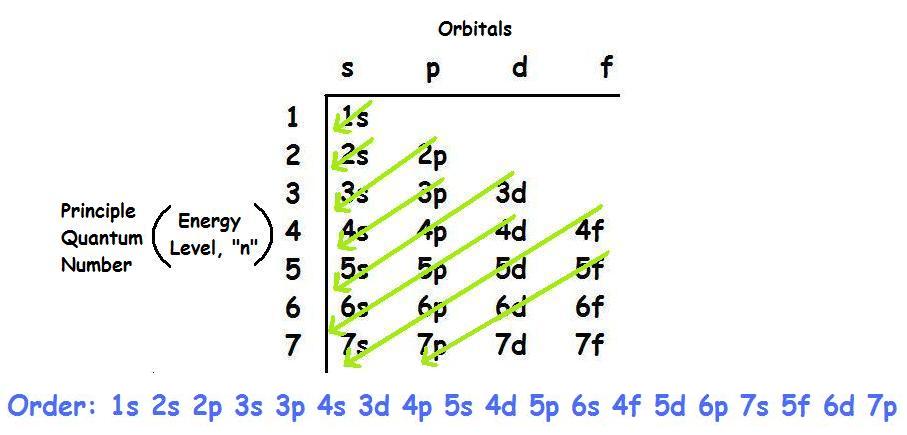
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f.
6s orbital. Each orbital has four nodal planes, all of which include the z-axis. It is defined as the three dimensional space where probability of finding electrons are maximum. Getting the correct order is a tricky business, but if you stick to the Aufbau principle (the first ordering you mentioned) you'll be pretty close with the guess.
They have the same maximum number of electrons. For a 6s orbital, n = 6. Thus, the energy gained by not shoving two electrons into the 6s orbital, plus that from a half-filled 5d subshell, is not enough to compensate for promoting an electron from 6s to 5d.
It may be simpler to think of these two letters in terms of orbital shapes (d and f aren't described as readily).However, if you look at a cross-section of an orbital, it isn't uniform. The spin quantum number of an electron must be either +1/2 or -1/2. Don't bet on it for d and f elements though.
The shape of the 6 s orbital. Revised Lone Pair model, in which the Bi-6s orbital interact with. And a d subshell (l = 2) consists of five orbitals, called d orbitals.
There are 118 elements in the periodic table. But still 6s fills first because aufbau rule states that electrons fill first in orbitals with lower energy (i.e. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f It is not necessary to memorize this listing, because the order in which the electrons are filled in can be read from the periodic table in the following fashion:.
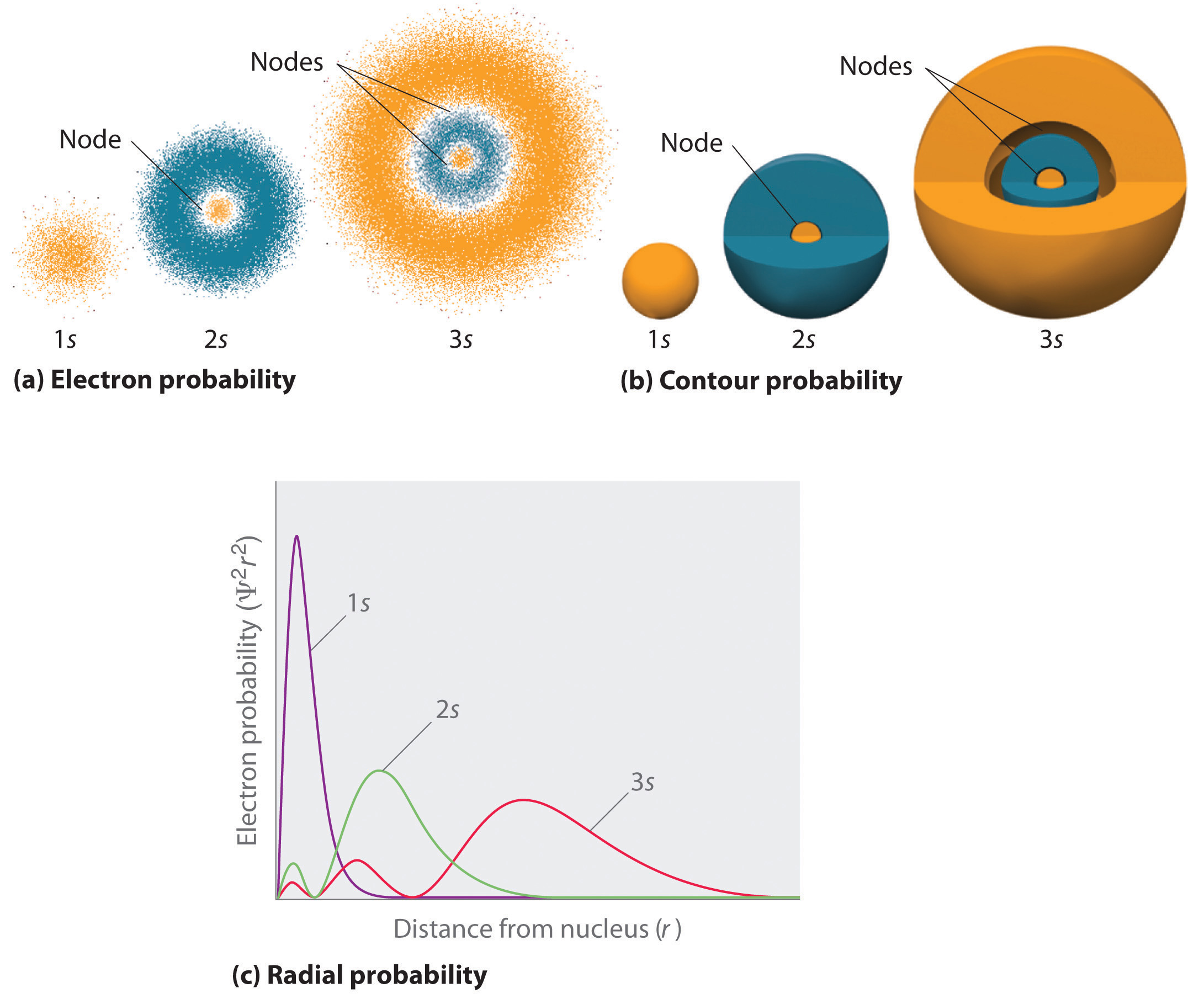
We have over 1500 academic writers ready and waiting to help you achieve academic success. For an atom with 118 electrons, the electron orbital configuration would be:. The ns orbital has (n-1) radial nodes, so the 6s-orbital has (6-1) = 5 nodes, as shown in the above plot.
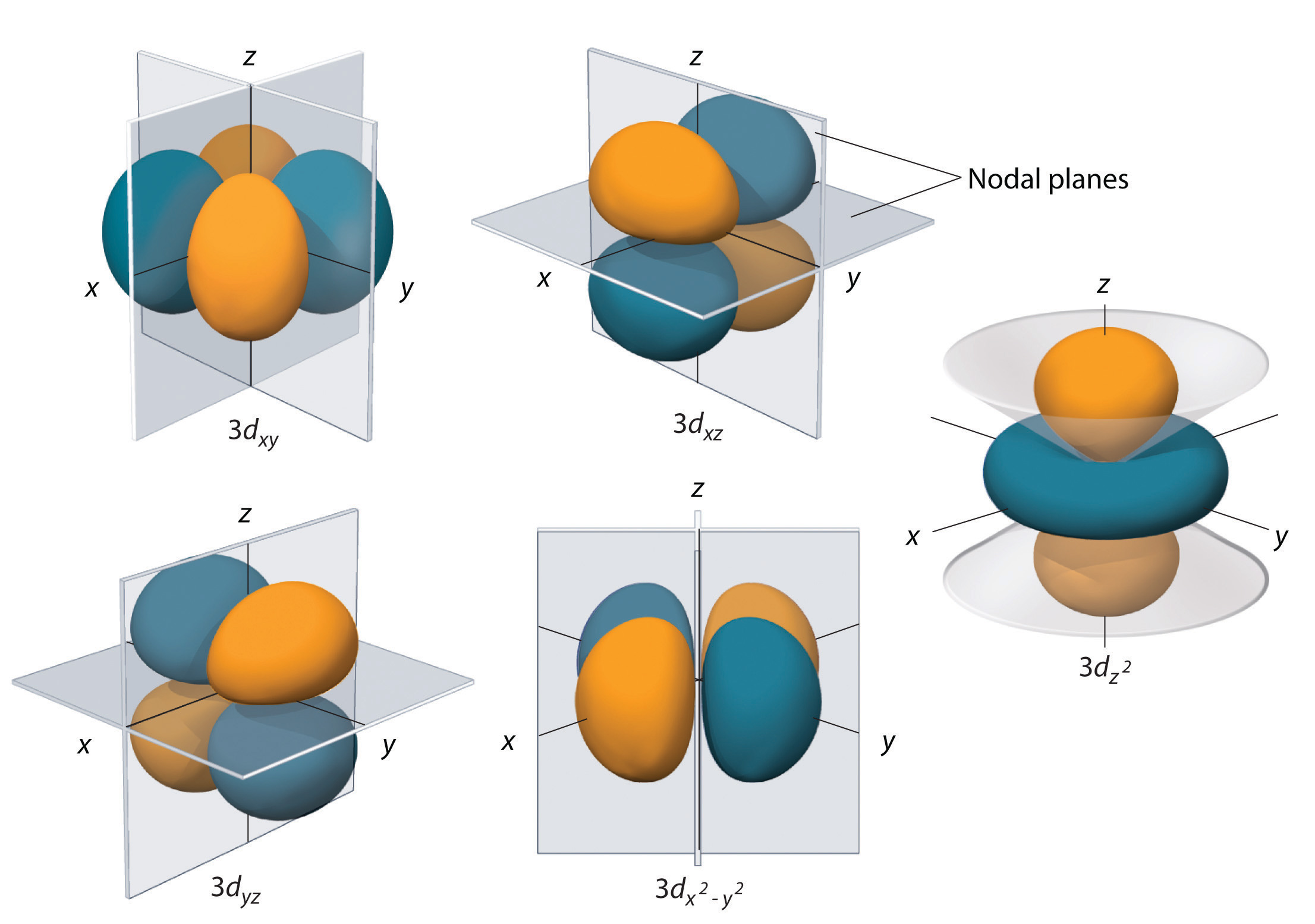
The 5p orbitals fill immediately after the 4d orbitals and immediately before the 6s because:-observed experimental results-theoretical calculations. At the first energy level, the only orbital available to electrons is the 1s orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital.
To be more. However, at the second level, there are also orbitals called 2p orbitals in addition to the 2s orbital. So, the most frequently used names for the s orbitals are 1s, 2s, 3s, 4s, 5s, 6s and 7s.
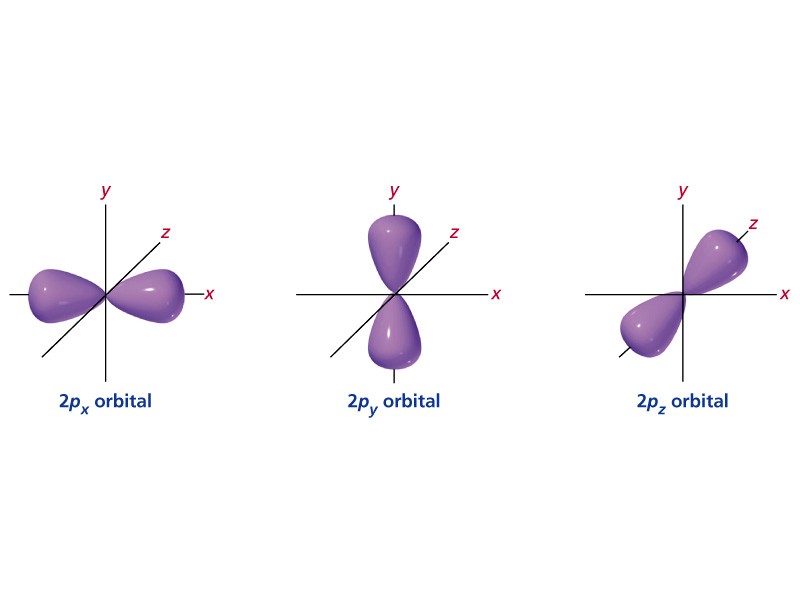
Get 1:1 help now from expert Chemistry tutors. The main difference between s orbitals is in the size. The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z).
According to quantum theory, the principal quantum number n specifies the size and energy level of the orbital. (i)It can be looked as that the 5d sub shell electrons are shielded better by the completely filled 4f sub shell electrons to make 5d to be lower energy than the 6s orbital. They are in the same energy level They have the same shape.
The 1s orbital is the smallest, and the 7s orbital is the largest. The quantum numbers n = 6 and l = 0 specify a 6s orbital. Rule for filling orbital:.
Order an Essay Check Prices. The 6 g zx 3 an abbreviation for 6 g x z ( x 2 - 3 y 2 ) and 6 g zy 3 an abbreviation for 6 g y z (3 x 2 - y 2 ) orbitals (next to bottom row in the image above) each. The individual orbitals are labeled with the magnetic quantum number, ml, which can take the 2l….
5s and 6s orbitals Check all that apply O None of these statements are true. This change of the trend has to be attributed exclusively to orbital effects;. Learn more about atomic orbital at Byjus.
Check all that apply. The origin of the spherical nodes becomes clearer upon examining the wave equation for this orbital. In the usual order of filling , the 6s orbital is filled before the 4f orbital.
A wave function for an electron in an atom is called anatomic orbital;. In the case of s-orbitals, there are a number of radial nodes that separate the largest, outer, component from the inner components. One orbital - 6s which can hold 2 electrons.
If the principle energy level is n=1 then the type of sublevel is 1sN=2---> type of sublevel is 2s and 2pN=3---> type of sublevel is 3s, 3p, and 3dN=4. Quantum Numbers describing Electronic Orbitals. Because of this effect, the 6s orbital is lower in energy relative to the 5d orbital than the 5s is relative to 4d or the 4s to 3d.
Xe 4f 14 5d 10 6s 2:. What is the total number of electrons possible in the 6s orbital?. There can be a total of six electrons in a "p" orbital.
How many unshared pairs of electrons are in this orbital diagram?. Each element has a unique atomic structure that is influenced by its electronic configuration, which is the distribution of electrons across different orbitals of an atom. For any atom there is just one 6 s orbital.
The number of nodes is related to the principal quantum number, n. 1s3- There is only one s orbital per energy level, and each orbital can hold a maximum of two electrons, so there can not be 3s electrons at any energy level. 4f is closer to nucleous and not 6s.
In the lanthanoid and actinoid atoms, the 4f and 5f spinors are significantly destabilized by the indirect relativistic effects. Because each orbital is different, they are assigned specific quantum numbers:. 2,3-Dimethylbutadiene and Acrolein(propenal) Quinone as Dienophile – Steroid Framework.
Observation of a smaller yet sizable interaction of Bi-6s and O-. That said, the orbital energies are not necessarily the same for all atoms. Xe 4f 6 6s 2:.
1s, 2s, 3s, 4s, 5s, 6s, 7s, 2p, 3p, 4p, 5p, 6p, 7p, 3d, 4d, 5d, 6d, 4f, 5f. Each orbital is spherical, with the nucleus at the center of the sphere. Working out which product is endo;.
This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.The term atomic orbital may also refer to the physical region or space where the electron can be. All atomic orbitals with n=10 are presented here.Note that the orbitals with negative m are identical to those with the same magnitude positive m value except for a rotation,and are not shown separately. O They have the same shape.
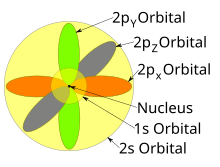
…orbital, which is called an s orbital;. Electron Configuration Chart for All Elements in the Periodic Table. A 2p orbital is more penetrating than a 2s, i.e., it has a higher electron density near the nucleus and inside the charge cloud of a 1s orbital.
For the 6 g x y ( x 2 - y 2 ) orbital, these planar nodes are the xz , yz , x = y and x = -y planes. The number of orbitals in a shell is the square of the principal quantum number:. In atomic theory and quantum mechanics, an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom.
An analogous transition occurs in silver, but the relativistic effects are smaller than in gold. List the sequence in which the following orbitals fill up:. Lower value of sum of principal quantum number and azimuthal quantum number).
There are 4 types of orbital:. This atomic orbital. LOGIN TO VIEW ANSWER.
The maximum number of electrons is the same Submit Request Answer. Since we know that energy of an electron is determined by its principal quantum number ‘n’ denotes the principal energy level of an electron. What is the maximum number of electrons allowed for the 6s orbital?.
The large orbital energy lowering in the 4f orbital of 64 Gd (4f 7) (5d 1) (6s 2) compared to that of (4f 8) (6s 2) might be responsible for the local minimum ratio(ε) of 64 Gd. Dont confuse with. ZOS-6S 6 inch Self-Generated Vacuum Random Orbital Sander.
The Principal Quantum Number (\(n\)) The principal quantum number, \(n\), designates the principal electron shell. The electron in the 6s orbital of Bi 3+ on the other hand extends to the periphery of the cation resulting in an overlap and hybridization with the anion orbitals. The distribution of electrons among the orbitals of an atom is called the electron configuration.The electrons are filled in according to a scheme known as the Aufbau principle ("building-up"), which corresponds (for the most part) to increasing energy of the subshells:.
Other articles where S-orbital is discussed:. This view is getting some support by the fact that the 6s orbital expands when proceeding beyond mercury, where the 6p orbitals are filled in. Of the four, we'll be concerned primarily with s and p orbitals because these are the most common in organic chemistry.
The Woodward Hoffman description of the Diels-Alder;. How many unshared pairs of electrons are in this orbital diagram. That on the right is sliced in half to show that there are five spherical nodes in the 6 s orbital.
An orbital can contain two electrons only if the electrons have opposite Which model allows a student to determine the total number of electrons in an atom and the electrons within Arsenic does not have any valence electrons in the 3d orbital because A - its 3d orbital is completely. Request Answer Submit Part B 3p and 4p orbitals Check all that apply. (click to see image?.
The orbitals are presented in six different ways, n and l versus m, n and m versus l, l and m versus n, n-l and l-m versus m, n-l and m versus l-m, and l-m and m versus n-l. Thus, the 6s orbital contraction might be well assigned to conventional orbital effects, in the first place. Asked by Wiki User.
OThey are in the same energy level. Part A 5s and 6s orbitals:. Get more help from Chegg.
The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. An atom is composed of subatomic particles, mainly, protons, electrons, and neutrons.Protons and electrons make the nucleus, which is located at the center of the atom.But electrons are positioned in orbitals (or energy levels) which are located outside the nucleus of an atom. Shapes of Orbitals and Electron Density Patterns.
A 3s orbital is even larger, and it has three nodes. O They have the same maximum number of electrons. Key Difference – 1s vs 2s Orbital Atom is the smallest unit of matter.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 The electron orbital configurations for atoms with less electrons will be truncated versions of this 118-electron configuration. The electronic transition from the 5d orbital to the 6s orbital is responsible for this absorption. 1s, 2s, 2p 3s, 3p,4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 1 5d 1 6s 2:. 1 2 = 1, 2 2 = 4, 3 2 = 9. After filling 6s, electrons would fill.
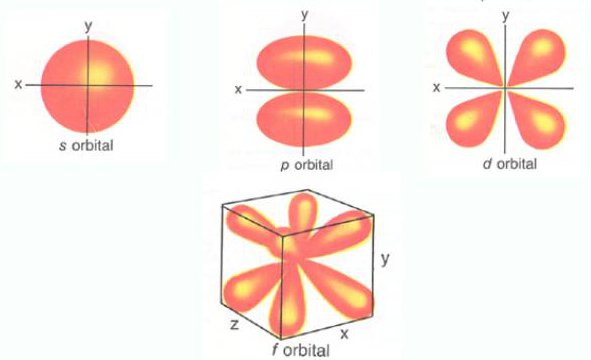
Orbitals Chemistry (s, p, d, and f Orbital) - Atomic Orbitals are of four different kinds, denoted s, p, d, and f, each with a different shape. Orbital explanation for the endo rule;. O-2p orbitals via th e empty Bi-6p orbitals.
A p subshell (l = 1) consists of three orbitals, called p orbitals;. In other words, all matter is made out of atoms. Intramolecular Diels-Alder – 1,3,9-decatrien-8-one;.
The secondary quantum number l specifies the shape of the orbital. S - orbital (maximum. The total number of electrons in a given atom is equal to its atomic number.
Each has its own specific energy level and properties. The image on the left is deceptively simple as the interesting features are buried within the orbital. Electrons cannot simply occupy.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 6 6s 2:. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2:. The electron in the 4f-orbital of Ce 3+ is relatively close (on average about –25 pm) to the nucleus and it is screened from the chemical environment by the 5s and 5p electrons.
Order Your Homework Today!. There are multiple orbitals within an atom. They have the same shape.
LOGIN TO POST ANSWER. Because n describes the most probable distance of the electrons from the nucleus, the larger the number n is, the farther the electron is from the nucleus, the larger the size of the orbital, and the larger the atom is.n can be any positive integer starting at 1, as \(n=1. To determine the orbital from a given pair which has higher energy in a many electron atom.

Color Online A The Pdos Of The Bi 6s Orbital Blue And Te 5p Download Scientific Diagram

Quantum Numbers

High School Chemistry Writing Electron Configurations Wikibooks Open Books For An Open World
What Are The Four Quantum Numbers Example

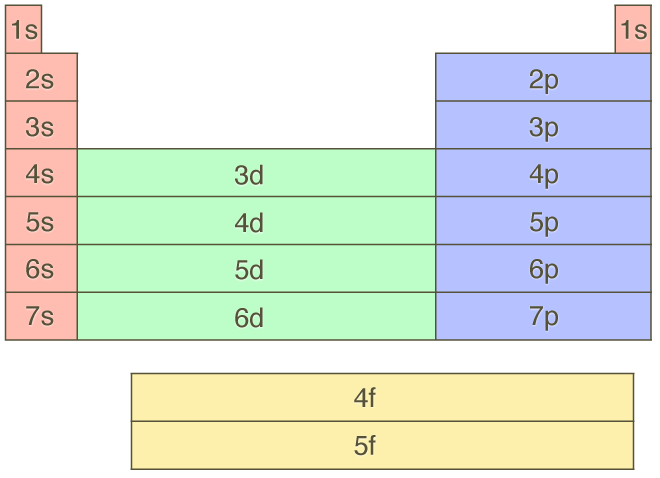
The Parts Of The Periodic Table
What Are The Possible Values Of N And Ml For An Electron In A 5d Orbital A N 5 And Ml 2 1 0 1 Or 2 B N
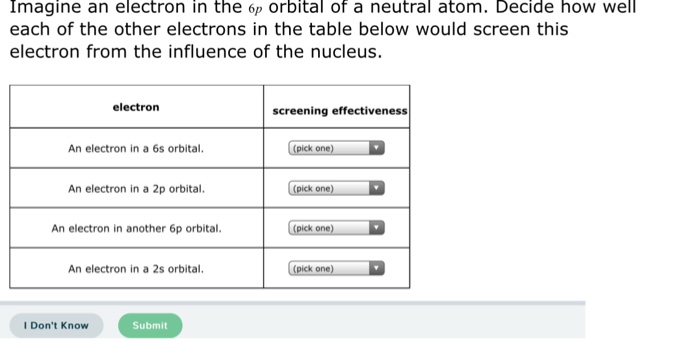
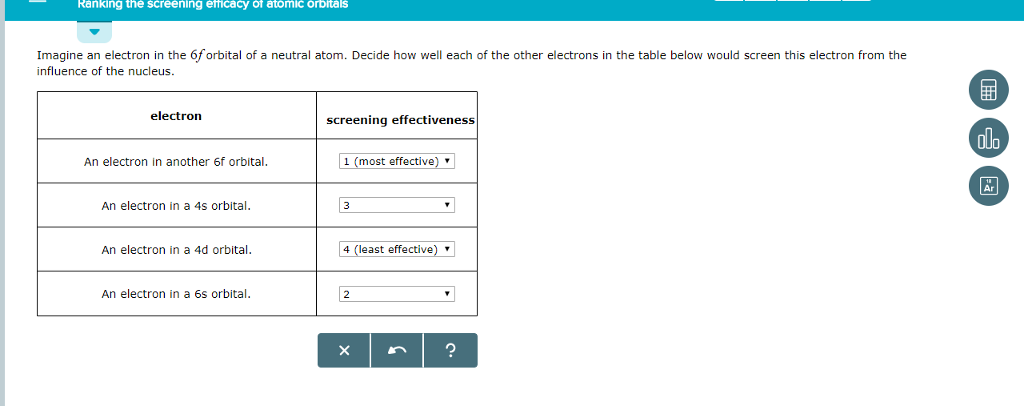
Solved Imagine An Electron In The 6p Orbital Of A Neutral Chegg Com

Imagine An Electron In The 6d Orbital Of A Neutral Atom Dec Clutch Prep
Solved Hi Can I Get Help In This Problem Hopefully You G Chegg Com

Chemistry The Central Science Chapter 6 Section 9

Strong Hybridization Between Bi 6s And O 2p Orbitals In Sillen Aurivillius Perovskite Bi4mo8x M Nb Ta X Cl Br Visible Light Photocatalysts Enabling Stable Water Oxidation Journal Of Materials Chemistry A
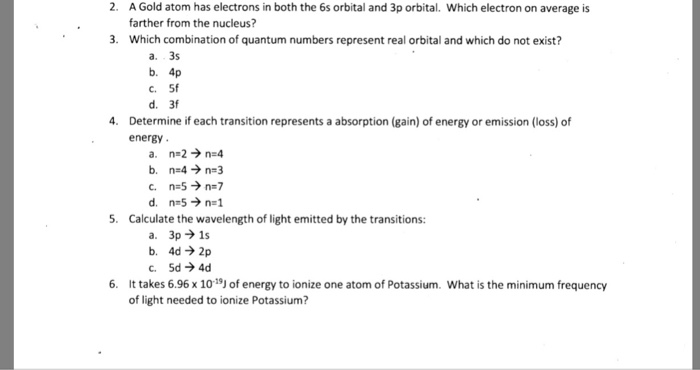
Solved A Gold Atom Has Electrons In Both The 6s Orbital A Chegg Com

Are Orbitals Always Filled In From Closest To Nucleus To Farthest Away Chemistry Stack Exchange

What Is The Total Number Of Electrons Possible In The 6s Orbital

Solved Not Sure Which Part I Got Wrong Here Is The Feedb Chegg Com
Shape 6s Atomic Orbital On White Stock Illustration

Statement 1 Among 5p And 6s 6s Orbital Have High Energy And Statement 2 N L For5p And Youtube

Di Tuujmcuous Use Of The Same When Electrons Are Added In The 6s Orbital What Happens To The Energy Level Of The 5d Orbitals As A Result After The Electron Enters 5d

Solved The Energy Required To Completely Remove The Outer Chegg Com
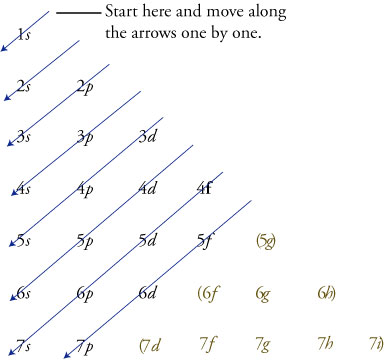
Electron Configurations
How To Answer Which Orbitals Are Possible For Ex 1p 2s 2p 3f Which Of The Following Are Possible Orbitals And Why Quora
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Electron Configuration Ppt Download

The Aufbau Principle And Hund S Rule Protocol

Color Online A The Pdos Of The Bi 6s Orbital Blue And Te 5p Download Scientific Diagram
Atomic Orbital Wikipedia

Promethium Atomic Structure Stock Image C018 3742 Science Photo Library

Relativistic Effects In Your Wedding Ring Sciences In The Mural Of Life

Electron Configurations Ppt Download

Quantum Number Wikipedia
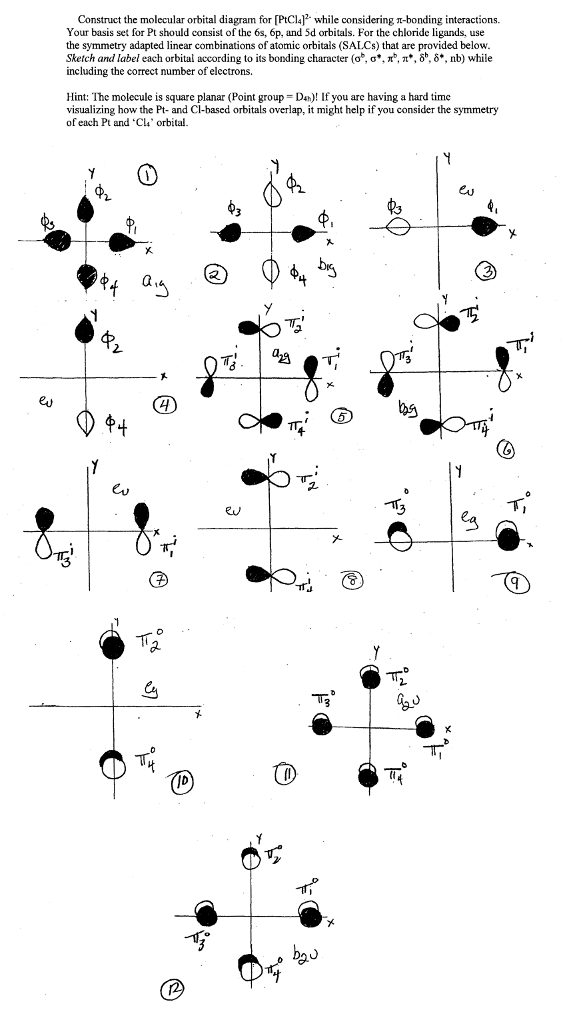
Solved Construct The Molecular Orbital Diagram For Ptcl4 Chegg Com

Cesium S Off The Map Valence Orbital Goesten 17 Angewandte Chemie International Edition Wiley Online Library

Cesium S Off The Map Valence Orbital Goesten 17 Angewandte Chemie Wiley Online Library
Dirk Bertels The Atom Filling The Gaps

Atomic Orbital Wikipedia

Perspective Relativistic Effects The Journal Of Chemical Physics Vol 136 No 15

A Electron Configuration And The Periodic Table

Webelements Periodic Table Periodicity Electronegativity Mulliken Jaffe P Orbital Period 6spd

The Relativistic Contraction Of The 6s Orbital And Expansion Of The 5d Download Scientific Diagram

Square Of The Radial Wavefunctions For The 4f 5s 5p And 6s Energy Download Scientific Diagram
Clarifying Electron Configurations Chemical Education Xchange
11 10 The Schrodinger Wave Equation For The Hydrogen Atom Chemistry Libretexts

The Calculated Homo And Lumo For The Free Pyridine Molecule And The 6s Download Scientific Diagram

Cesium S Off The Map Valence Orbital Goesten 17 Angewandte Chemie Wiley Online Library

Shape Of The 6s Atomic Orbital On White Background Stock Photo Picture And Royalty Free Image Image

Periodic Table The Basis Of The Periodic System Britannica
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry Libretexts
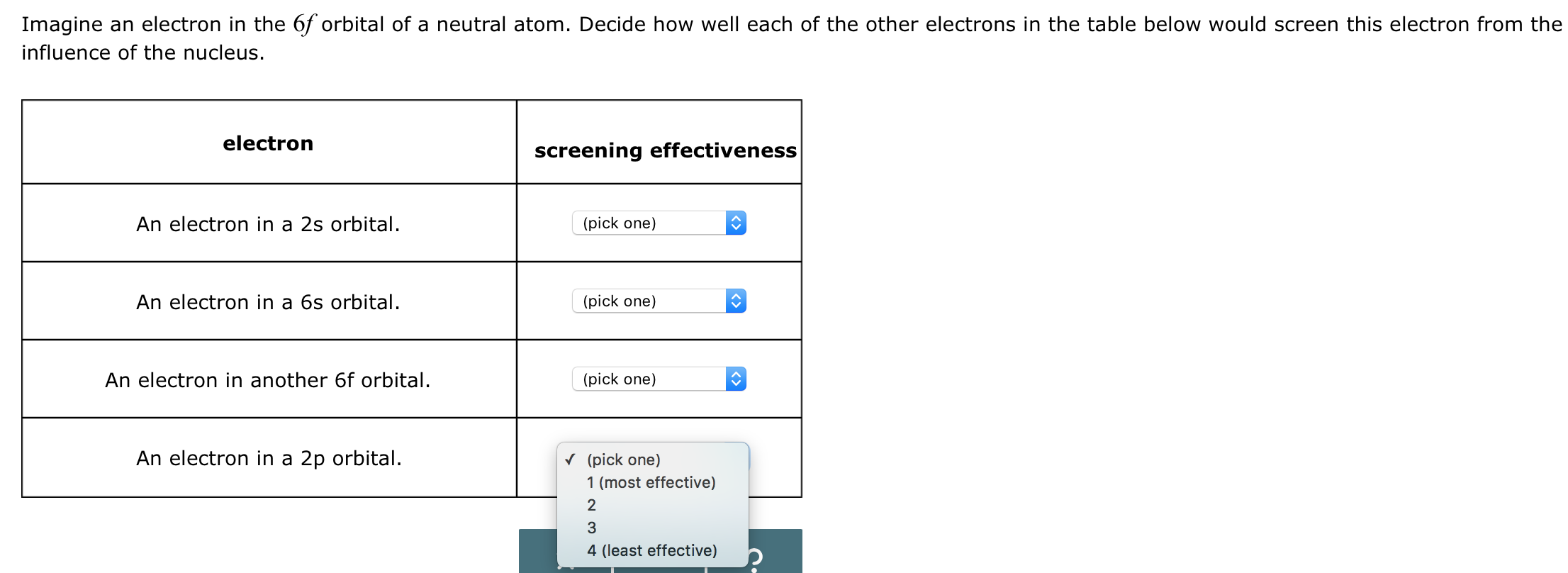
Solved Ranking The Screening Efficacy Of Atomic Orbitals Chegg Com
Building Up The Periodic Table

8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts

Relativistic Phenomena In The Chemistry Of The Platinum Group Metals Johnson Matthey Technology Review

Pdos On 2p Orbital Of C And 6s 5d Orbitals Of W 4c Atoms For Oc Vac Download Scientific Diagram

Square Of The Radial Wavefunctions For The 4f 5s 5p And 6s Energy Download Scientific Diagram

The Orbitron A Gallery Of Atomic Orbitals And Molecular Orbitals
Aufbau Principle
Q Tbn 3aand9gcrec2g1kkhkqfhs4nyclfjvy5cexshsc56npnn2zregi5c95fbk Usqp Cau
Atomic Orbital Wikiwand
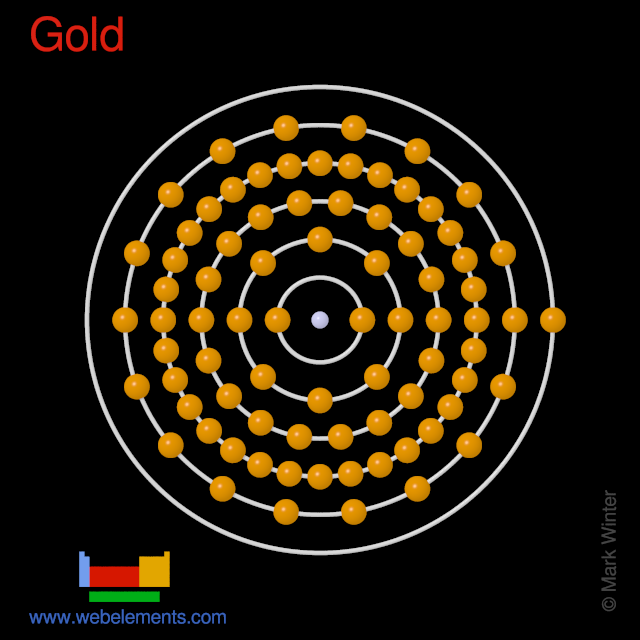
Webelements Periodic Table Gold Properties Of Free Atoms
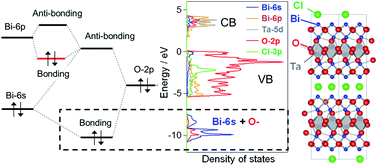
Strong Hybridization Between Bi 6s And O 2p Orbitals In Sillen Aurivillius Perovskite Bi4mo8x M Nb Ta X Cl Br Visible Light Photocatalysts Enabling Stable Water Oxidation Journal Of Materials Chemistry A
Which Sublevel Is Filled After The 5s Sub Level Socratic

Solved How Many Orbitals Does The 6s Subshell Have A 0 Chegg Com
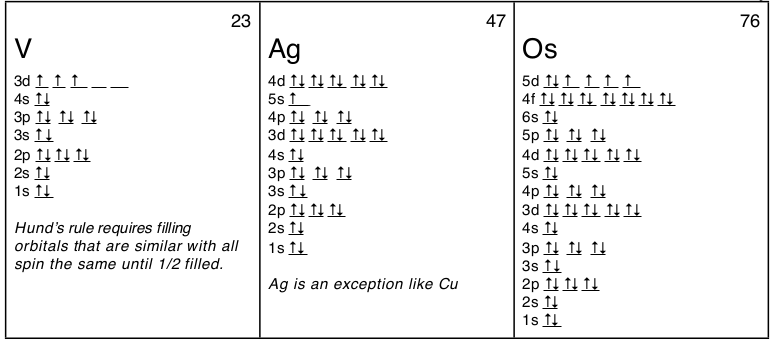
1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts
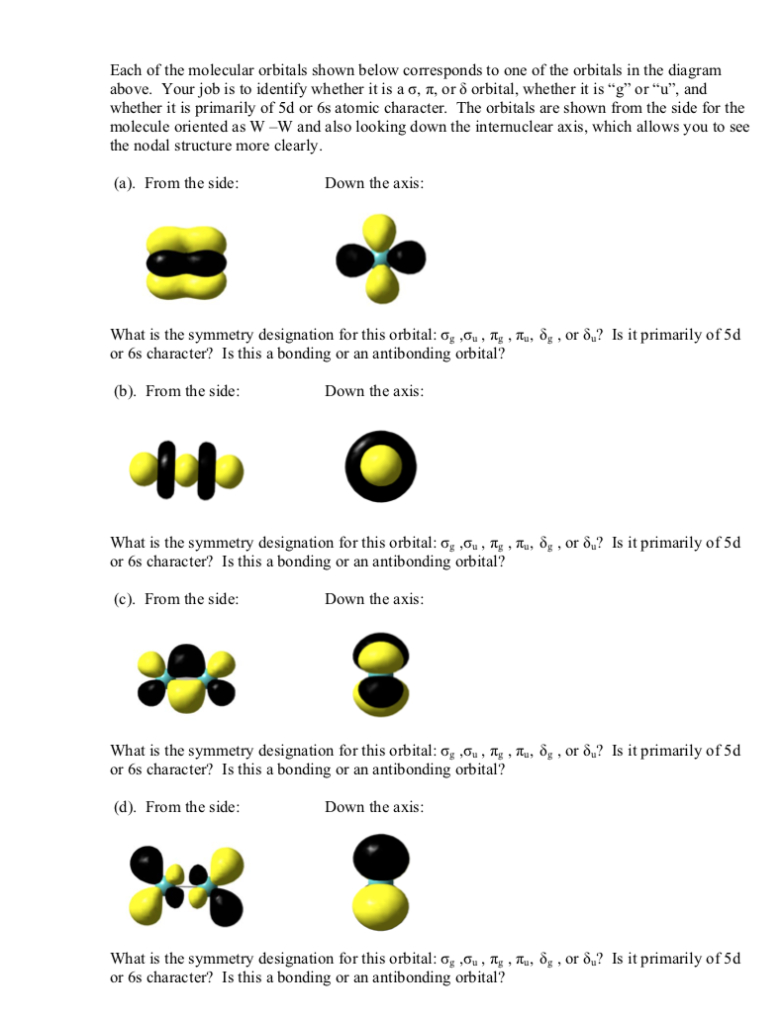
5 The Tungsten Dimer Molecule W2 Has The Molecu Chegg Com

17 The Valence Electronic Configuration Of An Atom Is 6s It D Orbital Of The Penultimate Shell Contains Two Unpaired Electrons Calculate The Atomic Number A 72 B 48 C 76 D 58
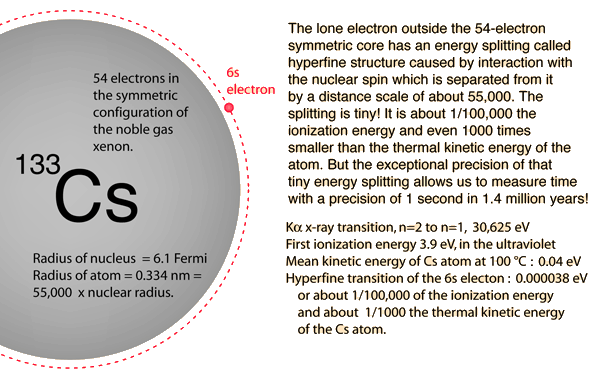
Atomic Clocks

Shape Of The 6s Atomic Orbital On White Background Available Stock Photo Picture And Royalty Free Image Image 5717

Quantum Mechanical Model Electron Configurations Ppt Download

Gamgi Screenshots

Shape Of The 6s Atomic Orbital On White Background Stock Photo Picture And Royalty Free Image Image

2 Calculated Relativistic Contraction Of The 6s Orbital The Download Scientific Diagram
Q Tbn 3aand9gcsj5l6seftrsq7znvqjaqk5xzpg9peezegqzql Brucb1cys9vg Usqp Cau
1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 7s 7p
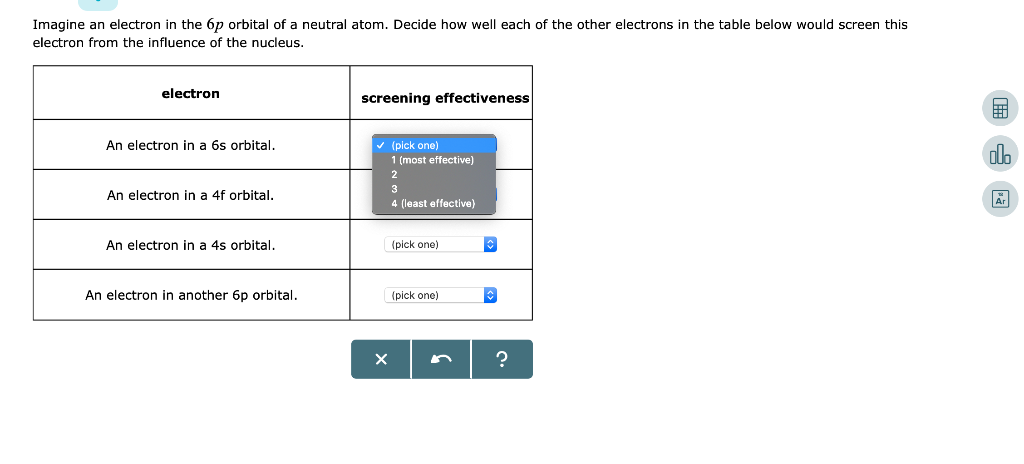
Solved Imagine An Electron In The 6p Orbital Of A Neutral Chegg Com

Aufbau Principle Wikipedia

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Atomic Orbital Wikipedia
What Is The Ground State Electron Configuration Of The Element Kr Socratic

A Molecular Orbital Plots Of Ir 5d Orbitals In R 3 Irf 8 B Download Scientific Diagram
The Order Of Filling 3d And 4s Orbitals

Gaussian Basis Sets For The Calculation Of Some States Of The Lanthanides

A Computational Investigation Of Orbital Overlap Versus Energy Degeneracy Covalency In Ue 2 2 E O S Se Te Complexes Dalton Transactions Rsc Publishing Doi 10 1039 C9dta
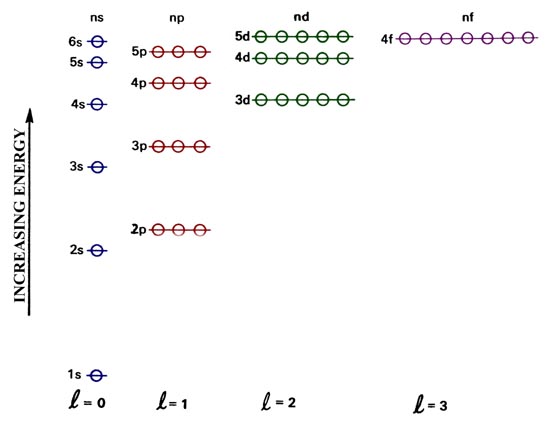
Many Electron Atoms The Electronic Basis Of The Periodic Table

Solution A Certain Electron In An Atom Is Clutch Prep
Q Tbn 3aand9gcrbzo9xp8ge974waopl5plarfs6new9mqhak1ynxvkbdjcpdetx Usqp Cau

Electron Configuration Wikipedia
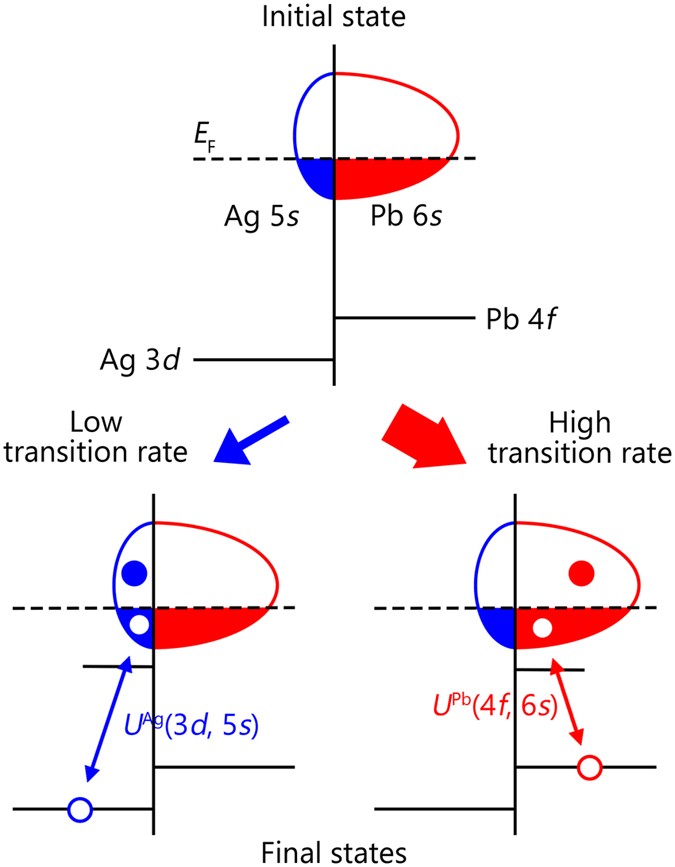
Element Specific Orbital Character In A Nearly Free Electron Superconductor Ag 5 Pb 2 O 6 Revealed By Core Level Photoemission Scientific Reports

Solved Select All That Are True For Neutral Atoms The 4 Chegg Com
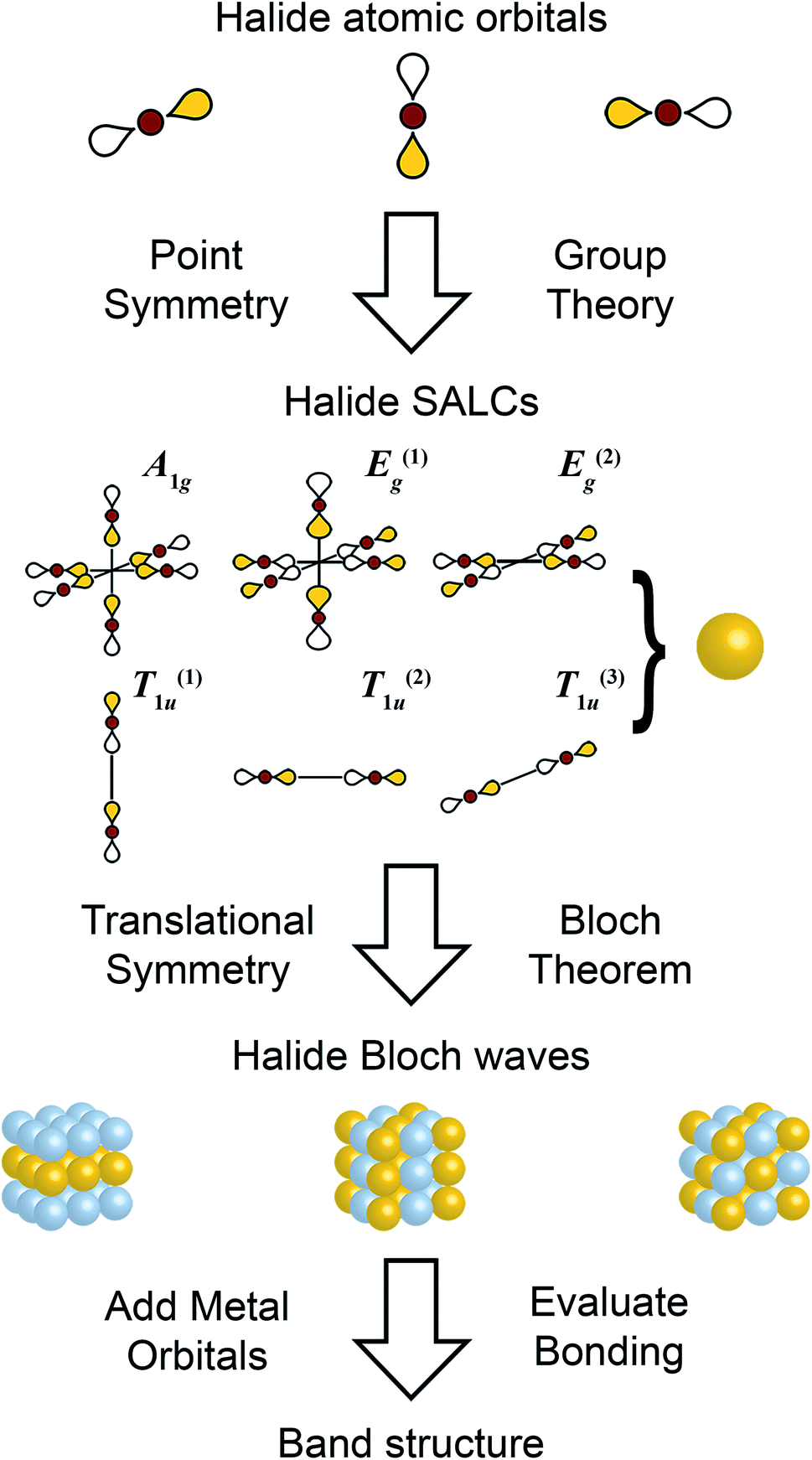
A Pencil And Paper Method For Elucidating Halide Double Perovskite Band Structures Chemical Science Rsc Publishing Doi 10 1039 C9scc

Solved A Two Types Of Nodes Occur In Atomic Orbitals Sp Chegg Com
Q Tbn 3aand9gcq6pcocjmyoorqurs8vizjbtxloj2nxidvntygwlup0ifa9h0wg Usqp Cau

It Fulus M Uus Used For The Same B When Electrons Are Added In The 6s Orbital What Happens To The Energy Level Of The 5d Orbitals As A Result After The

Illustration Of The Orbital Interactions That Lead To Lone Pair Download Scientific Diagram

Atomic Orbital Wikipedia

Electron Configuration Wyzant Resources

Why Does Platinum S Electron Configuration Not Conform To Hund S Rule I E Have A Full 5d Orbital As Other Metals In Its Group Do E G Palladium Quora
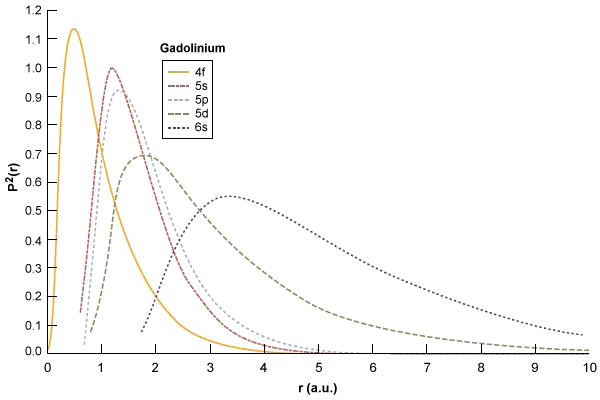
The Lanthanides

Figure 6 From Wannier Orbital Overlap Population Woop Wannier Orbital Position Population Wopp And The Origin Of Anomalous Dynamical Charges Semantic Scholar
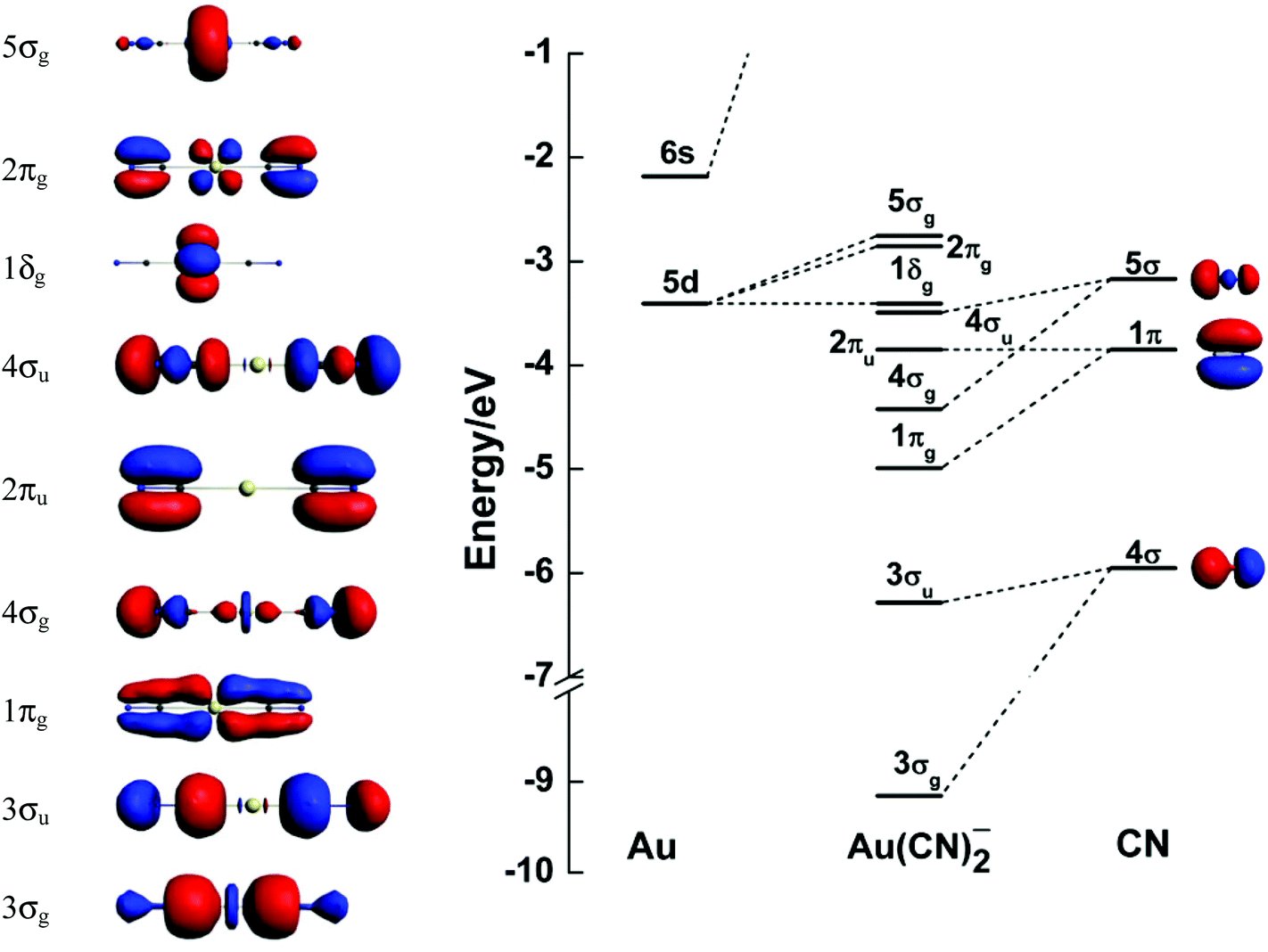
On The Gold Ligand Covalency In Linear Aux2 Complexes Dalton Transactions Rsc Publishing



