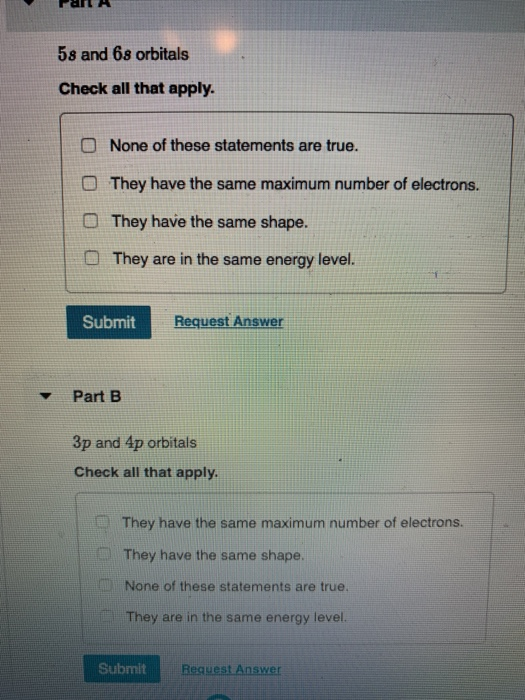
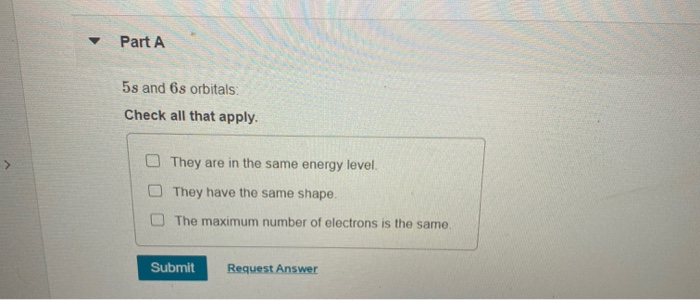
5s And 6s Orbitals
Solved For After Each Digram Indicate The Number Of Unpai Chegg Com

01 The Increasing Order Of Energy Of Orbitals Is 1 4f 6p 6d 2 4d 5s 5p In 3 5d 41 6s 4 6p 5d 6d

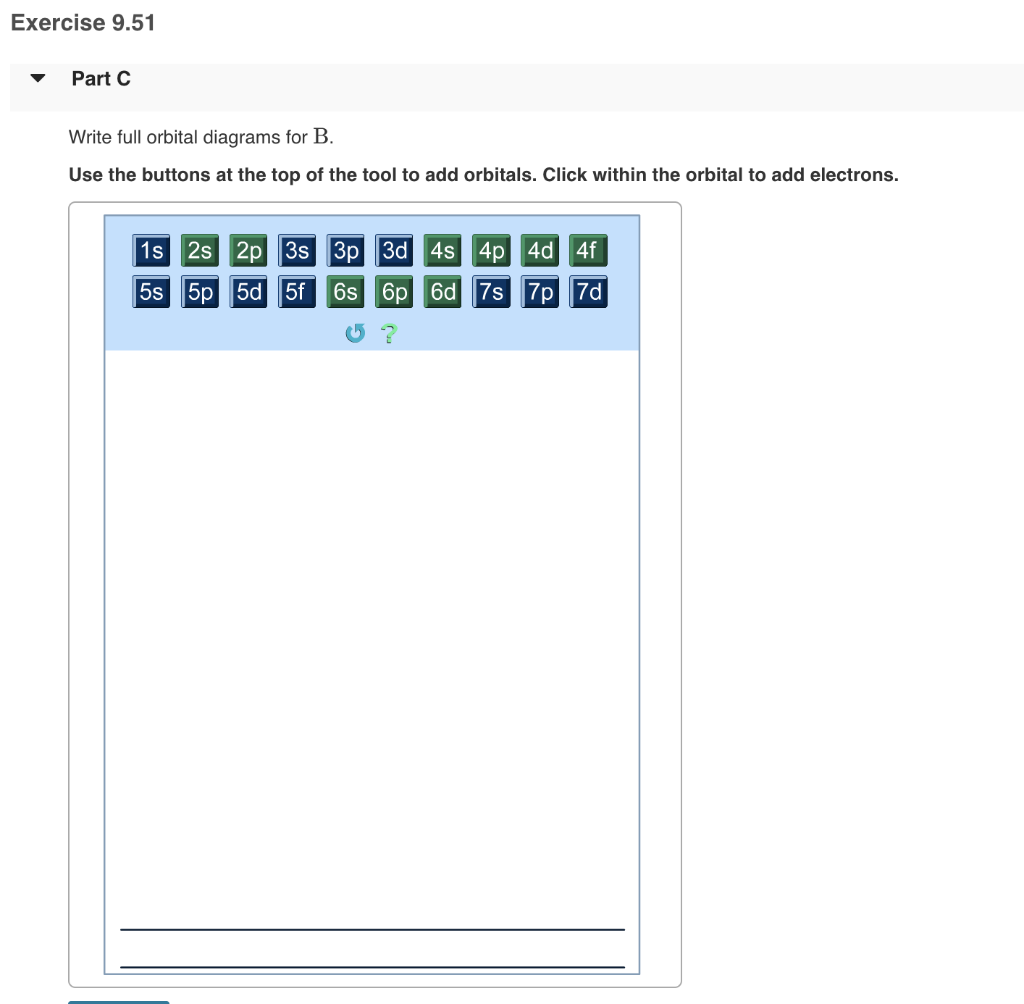
Part A Part B Part C Part D Hg2 Use The Buttons At The Top Of The Tool To Add Orbitals In Order Of Increasing Energ Homeworklib
Solved 5s And 6s Orbitals Check All That Apply O None Of Chegg Com
Clarifying Electron Configurations Chemical Education Xchange
Ppt Electron Configurations And Orbital Diagrams Powerpoint Presentation Id
Then, we optimised 6s and 6p sep-, arately by xing the orbitals obtained previously.

5s and 6s orbitals. (a) 1s 2s 2p 3s (b) 3s 3p 4s 4d (c) 4d 5p 6s 4f 5d (d) 7s 5f 6d 7p II. The four different types of orbitals (s,p,d, and f) have different shapes, and one orbital can hold a maximum of two electrons. Orbitals that have the same value of the principal quantum number form a shell.Orbitals within a shell are divided into subshells that have the same value of the angular quantum number.
Request Answer Submit Part B 3p and 4p orbitals Check all that apply. The s orbitals are spherical, while p orbitals are polar and oriented in particular directions (x, y, and z). 4 orbitals (s, p, d, f) 2.
Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. At element 118, the orbitals 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, 5f, 6s, 6p, 6d, 7s and 7p are assumed to be filled, with the remaining orbitals unfilled. Please see the diagrams at What are the shapes and designations of the f orbitals?.
The s sublevel has only one orbital and can therefore hold only two. For both crystals, the top of the valence band is found to consist mainly of the σ-antibonding states of Pb 6s and I 5p orbitals, and the bottom of the conduction band to be composed primarily of the σ-antibonding states of Pb 6p and I 5s orbitals. What is the total # of orbitals in n = 4 E.L.?.
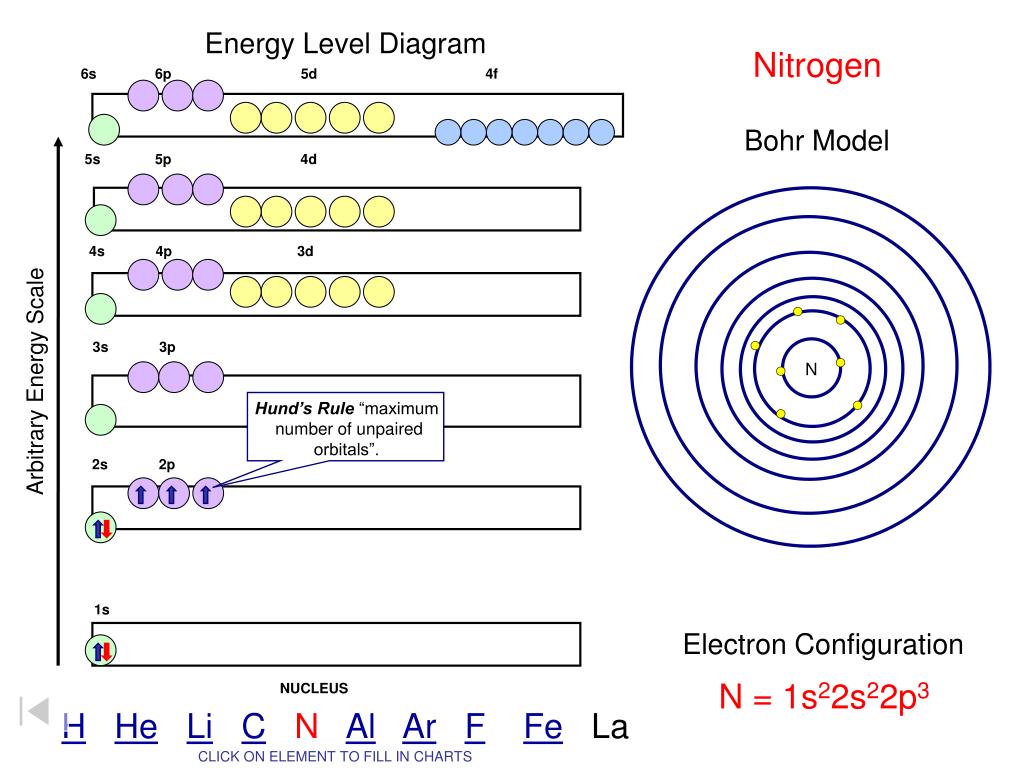
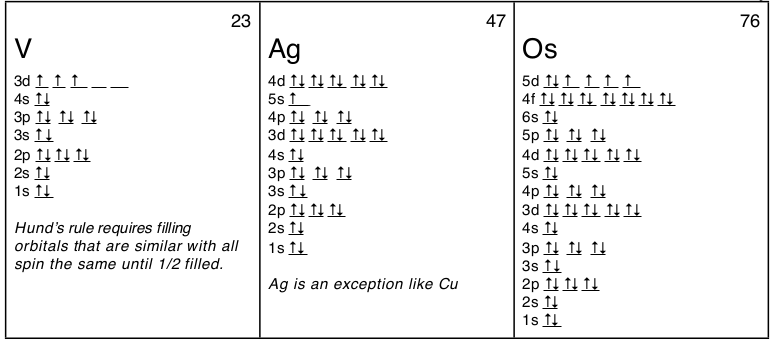
Orbitals can hold no more than two electrons each. An atom has a nucleus of protons and neutrons. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible.
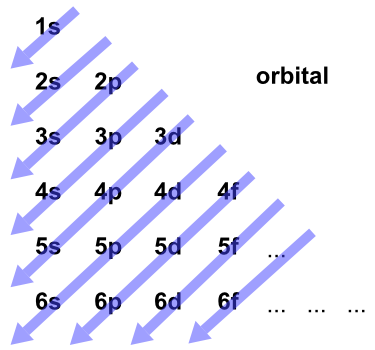
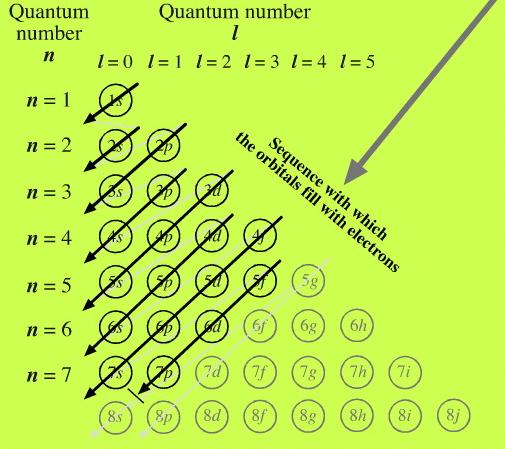
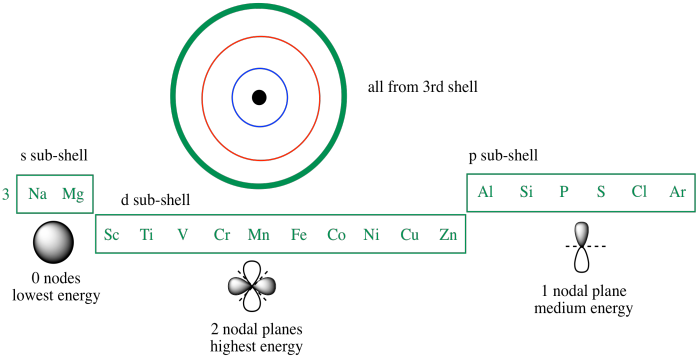
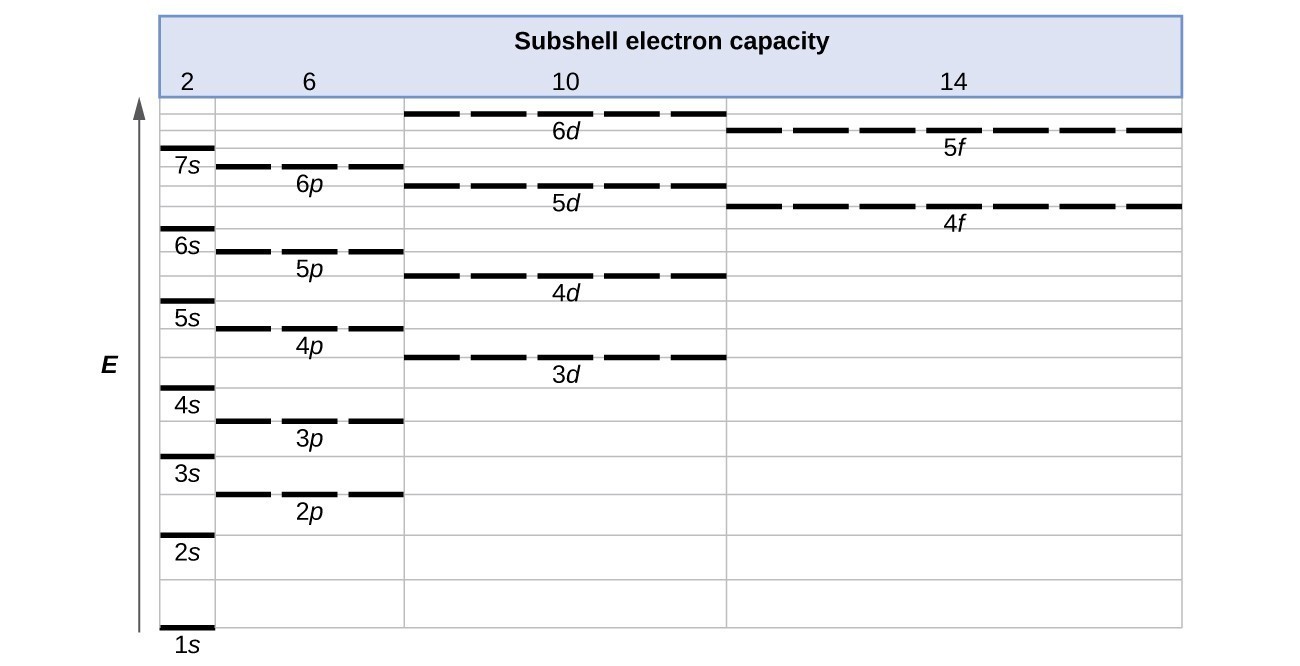
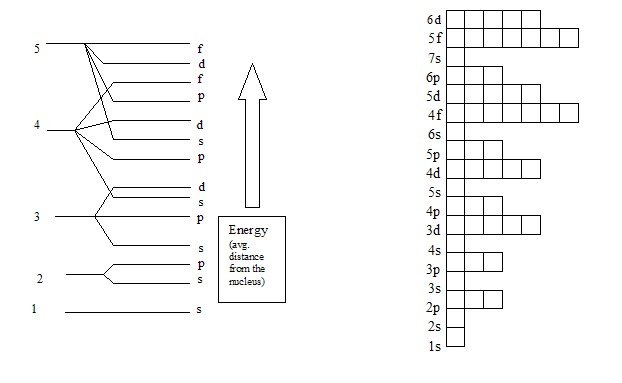
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d… To help you, here is the above list with the orbitals included. 1s s 2p 3s 3p 4s 3d 4p 4d 4f n = 3 n = 4 Energy level (n) Energy sublevel Increasing Energy. The diagram (not to scale) summarises the energies of the orbitals up to the 4p level.
5p, 5d, 5f, 6s, 6p Solution I. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d … This order corresponds to the order in which the energy sublevels are filled by electrons. Chemists describe the shell and subshell in which an orbital belongs with a two-character code such as 2p or 4f.The first character indicates the shell (n = 2 or n = 4).
For example, Pt metal must be promoted from the 6s 1 5d 9 atomic ground state to 6s 1 5d 7 6p 2 in order to make six bonds per atom, and the energy cost of promoting electrons from the 5d to the 6p orbitals is reflected in the net bonding energy. The order of filling of electrons in orbitals is 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d . 3)d- and s- orbitals can hold maximum of 10 and 2 electrons respectively.Thus , the number of valence electrons in these elements ranges from 3- 12.Generally the group number tells us the number of valence electrons in that group, but with. Shells and Subshells of Orbitals.
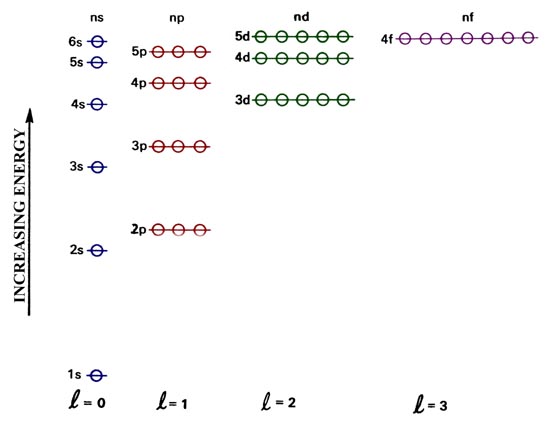
And 4fN=5---> type of sublevel is 5s, 5p, 5d, 5f and 5gN=6---> type of sublevel is 6s, 6p, 6d, 6f, 6g, and 6hN=7---> type of. 4d, 4f, 5s, 5p (b) Which of the following orbitals has the highest energy?. 5s and 6s orbitals.
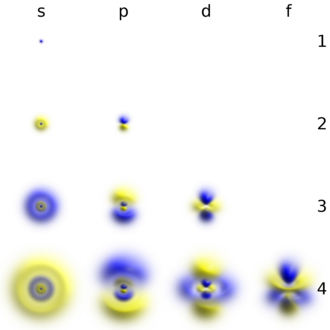
In this printable math activity, students will multiply by 6s in order to fill out all the bubbles on the multiplication caterpillar. Orbitals on different energy levels are similar to each other, but they occupy different areas in space. It may be simpler to think of these two letters in terms of orbital shapes (d and f aren't described as readily).However, if you look at a cross-section of an orbital, it isn't uniform.
Part A 5s and 6s orbitals:. The 1s orbital is the smallest, and the 7s orbital is the largest. Notice that atomic numbers 57 through 70 on the periodic table below are in the 4f portion of the table.
Get more help from Chegg. The p subshell (ℓ = 1) contains three orbitals (in some systems, depicted as three "dumbbell-shaped" clouds), so the m ℓ of an electron in a p orbital will be −1, 0, or 1. In other words, all matter is made out of atoms.
Lower the value of (n + l), lower is the energy. Step 3 (3) The energy levels are determined with the help of principal quantum number (n). O They have the same maximum number of electrons.
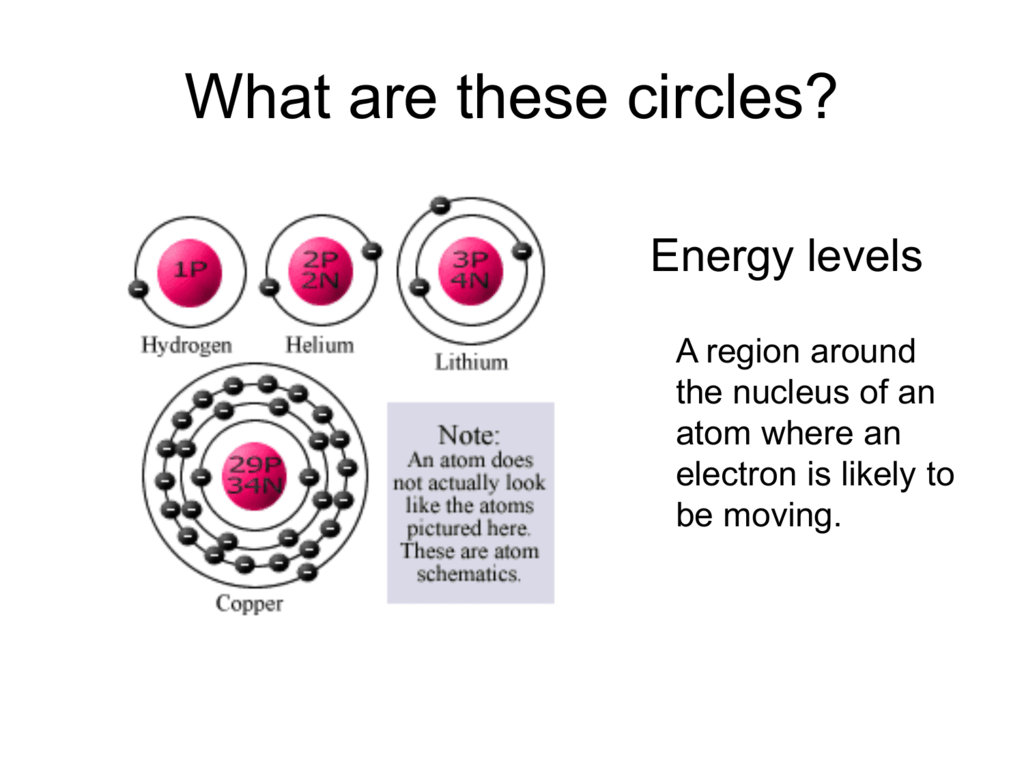
REPRESENTING ELECTRON LOCATION 3. If the principle energy level is n=1 then the type of sublevel is 1sN=2---> type of sublevel is 2s and 2pN=3---> type of sublevel is 3s, 3p, and 3dN=4. An atom is composed of subatomic particles, mainly, protons, electrons, and neutrons.Protons and electrons make the nucleus, which is located at the center of the atom.But electrons are positioned in orbitals (or energy levels) which are located outside the nucleus of an atom.
6d 6d 5f 7s5f 7s 6p 6p 5d 5d 4f 4f 6s 6s5p 5p 4d 4d5s 5s 4p 4p 3d 4s3d 4s 3p 3p 3s 3s 2p 2p 2s 2s 1s 1s 7s 6s 5s 4s 6d 6p 5p 4p 3p 3s 2s 1s 2p 5d 4d 3d 5f 4f Application • MRI (Magnetic Resonance Imaging) Developed by Raymond Damadian Based on the magnetic fields produced by atoms. 4s 3d 4p 5s 4d 5p 6s 4f Write a general rule to describe the filling of orbitals in an atom. Quantum numbers – give the set of four QN for each electron in the 3s orbital in a sodium atom.
Li, Na, K, Rb, Cs and Fr receive the last electron in 2s, 3s, 4s, 5s,6s and 7s orbitals. 3rd through 5th Grades. Electrons will occupy different orbitals in a given subshell, before two electrons will occupy a single orbital.
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f. When orbitals of identical energy are available, electrons first occupy these orbitals singly with parallel spins rather than in pairs (Hund’s rule). They are in the same energy level They have the same shape.
What does an atom look like?. In the box to the right, enter the symbol of first element in the periodic table to have two completed sets of d-orbitals:. (a) 5s (b) 5f Question 3 When 3d orbital is complete, the new electron will enter the (a) 4p-orbital.
The order of filling orbitals. The d subshell (ℓ = 2) contains five orbitals, with m ℓ values of −2, −1, 0, 1, and 2. 3s and 4s sublevels.
Have a higher energy in B than in A Feedback For 2s, n+ =2+0=2. A simple extrapolation from the Aufbau principle would predict the eighth row to fill orbitals in the order 8s, 5g, 6f, 7d, 8p;. Thanks for the A.
There is a simple way of remembering how electrons fill up orbitals, shown in the accompanying diagrams:. Get 1:1 help now from expert Chemistry tutors. Understanding it will help in gaining a better understanding of the concept of quantum numbers and their applications in physics and chemistry.
They have the same shape and the same maximum number of protons. You would observe that there are a lot of ball like probability density functions of the electron pair in the f-orbitals. They have the same maximum number of electrons.
Shapes of Orbitals and Electron Density Patterns. Photoelectron spectra of the valence-band region indicate that the electronic structures change. Only orbital notation shows the orbitals.
The energy levels for 5s and 6s orbitals is 5 and 6 respectively. In other words, electrons will occupy the lowest-energy orbital first before filling higher-energy orbitals. 1s, 2s, 3s, 4s, 5s, 6s and 7s orbital.These elements also belong to s block and have ns 2 as.
There are many exceptions to this order among the d- and f- Transition Elements since (n+2)s, (n+1)d, and (n)f orbitals are close in energy when n is large. For the one electron, the QN would be 3, 0, 0, +1/2. 5s and 6s orbitals b.
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s. When orbitals have the same energy level, each orbital gets one electron before any orbital gets two electrons.
The arrangement of orbitals on the basis of energy is based upon their (n +l) value. (5s and 6s Only) Practice multiplication facts focusing on 5s and 6s with this printable cut-out activity. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d In the box to the right, enter the symbol of first element in the periodic table to have 9 s-electrons:.
A set of p orbitals or a complete sublevel such as the 3p sublevel can have a maximum of 6 (2 times 3) electrons. The total of the superscripts in an element's electron configuration equals. Key Difference – 1s vs 2s Orbital Atom is the smallest unit of matter.
But after element 1, the proximity of the. There is one electron in the 3s orbital in a sodium atom. The 5s and 6s orbitals carry two electrons each , 3p and 4p orbitals carry 6 electrons each and 3s and 4s orbitals carry 2 electrons each and 2s and 2p do not carry same number of electrons.
Based upon the above information, arrange the following orbitals in the increasing order of energy. The rest of the elements i.e. A 25, 275 (where "I" means the neutral atom) :.
The electronic configuration of iodine is 5s 2 5p x 2 5p y 2 5p z 1. A single p orbital can have a maximum of 2 electrons. N=1 as an integer.
The lowest energy level electron orbitals are filled first and if there are more electrons after the lowest energy level is filled, they move to the next orbital. 3p and 4p orbitals. They have the same shape.
We begin therefore by showing in the left-hand columns of Table I the configurations given by Martin et al. OThey are in the same energy level. In other words, when we.
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. One orbital - 6s which can hold 2 electrons. The following is a list of the sublevels from lowest to highest energy:.
The maximum number of electrons is the same. So, the electron pair. Sequence the following orbitals in the order that they should fill up according to the Aufbau Principle:.
For orbitals having the same values of (n + I), the orbital with lower value of n will have lower energy. All s orbitals have a spherical shape. They have the same shape.
5s and 6s orbitals Check all that apply O None of these statements are true. For the odd-con n Agurations, we, rst optimised the spectroscopic orbitals from 1s to 5d on the ground con n Aguration. 5s 5p 5d 5f 6s 6p 6d 6f.
Sets of p orbitals are a criss-crossing set of three "dumbbell" shaped lobes. The maximum number of electrons is the same Submit Request Answer. Be, Mg, Ca, Sr, Ba and Ra.The last electron in these elements also enter the s orbital i.e.
Asked by Wiki User. The main difference between s orbitals is in the size. Each orbital is spherical, with the nucleus at the center of the sphere.
The orbitals were op-timised in several steps. Sublevels in n=3 as a integer. Because of their strong bonding energy, elements in the middle of the 4d and 5d series have very.
According to Ground-state configurations of ionic species I through XVI for Z = 57-74 and the interpretation of 4d-4f emission resonances in laser-produced plasmas Phys. 6d 1s 2s 3s 4s 5s 6s 2p 3p 4p 5p. Orbitals fill in the following order:.
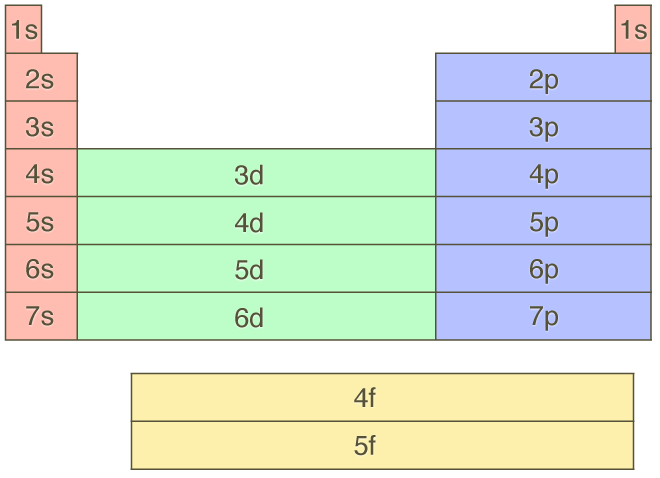
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6 This table gives a comprehensive list of the electron distribution in all elements. For even-parity, all orbitals, from 1s to 6p, were optimised together. According to the aufbau principle, the order of the filling of orbitals is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p… The IUPAC defines the aufbau principle as:.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6. The p, d, and f orbitals have different sublevels, thus can hold more electrons. So, the most frequently used names for the s orbitals are 1s, 2s, 3s, 4s, 5s, 6s and 7s.
O They have the same shape. (a) Which of the following orbitals has the lowest energy?. It is a common mistake to forget that the 4f sublevel is filled after the 6s sublevel and before the 5d sublevel.
The distribution of electrons among the orbitals of an atom is called the electron configuration.The electrons are filled in according to a scheme known as the Aufbau principle ("building-up"), which corresponds (for the most part) to increasing energy of the subshells:. For lanthanum through lutetium. The order of filling is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s.
The order of the electron orbital energy levels, starting from least to greatest, is as follows:. Also, the s orbitals occur singly.

Electron Configuration Anomalies Villanova College Chemistry Blog
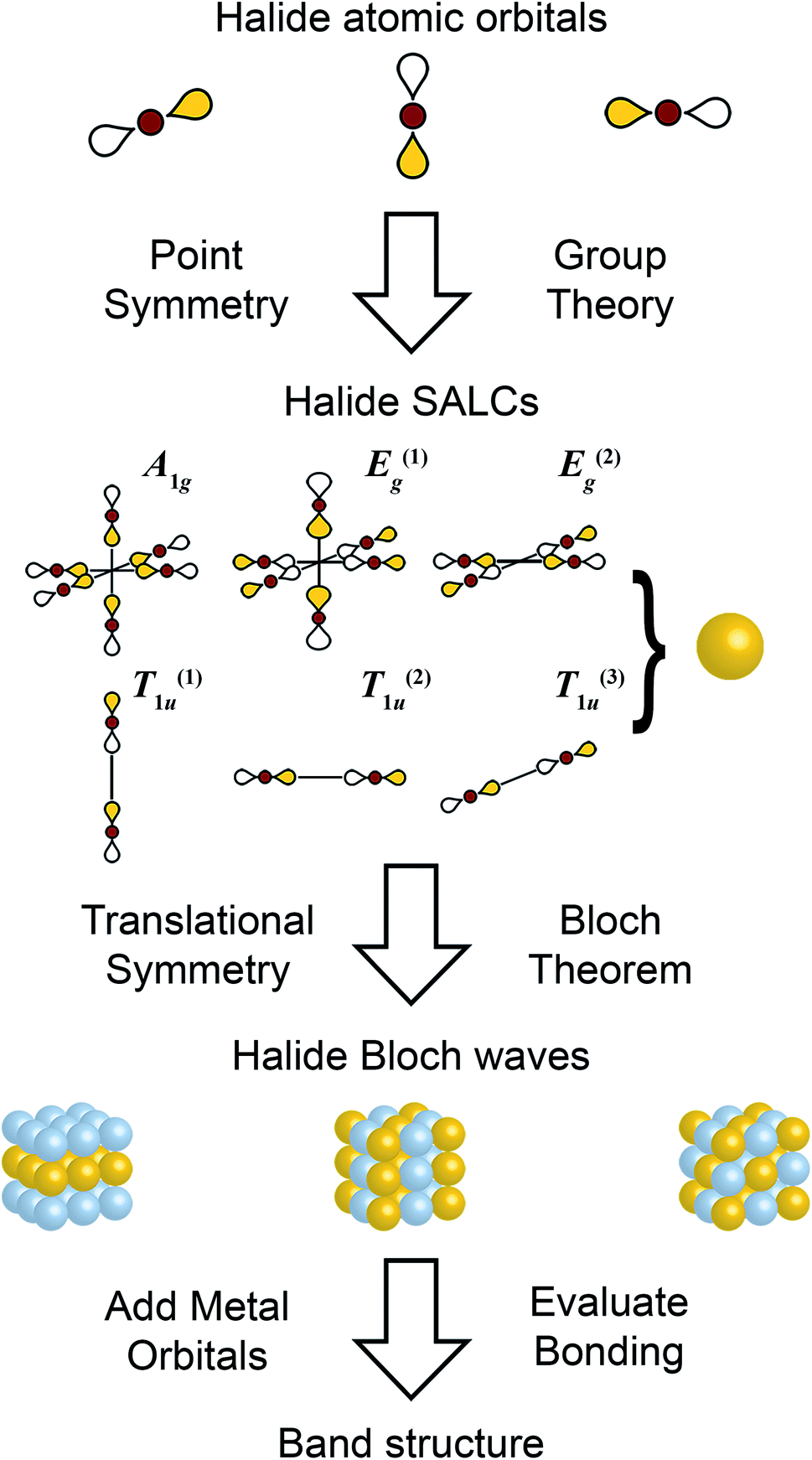
A Pencil And Paper Method For Elucidating Halide Double Perovskite Band Structures Chemical Science Rsc Publishing Doi 10 1039 C9scc
The Lanthanides

Solved 4 Arrange The Orbitals For A Many Electron Atom I Chegg Com
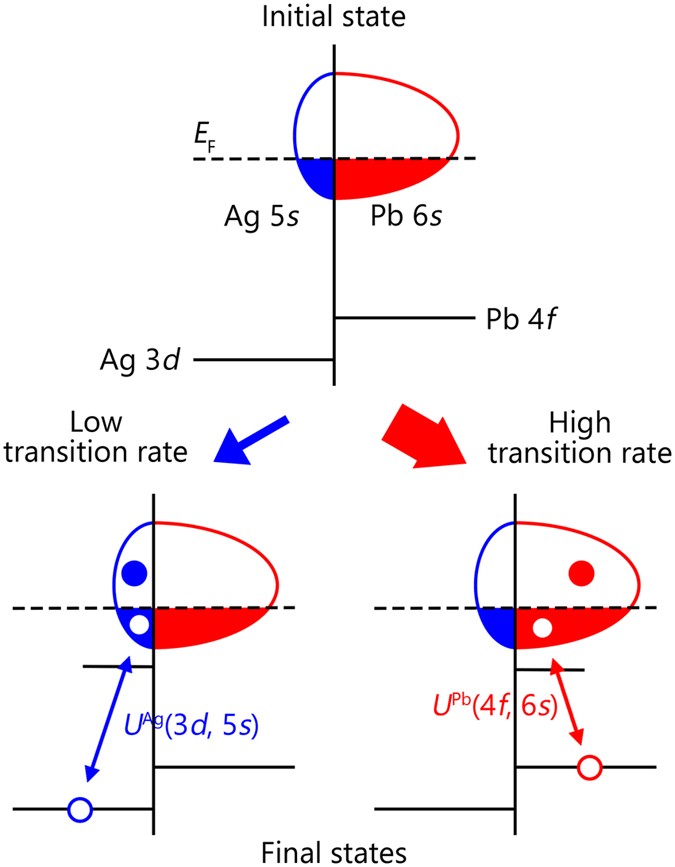
Element Specific Orbital Character In A Nearly Free Electron Superconductor Ag 5 Pb 2 O 6 Revealed By Core Level Photoemission Scientific Reports

Cesium S Off The Map Valence Orbital Goesten 17 Angewandte Chemie Wiley Online Library
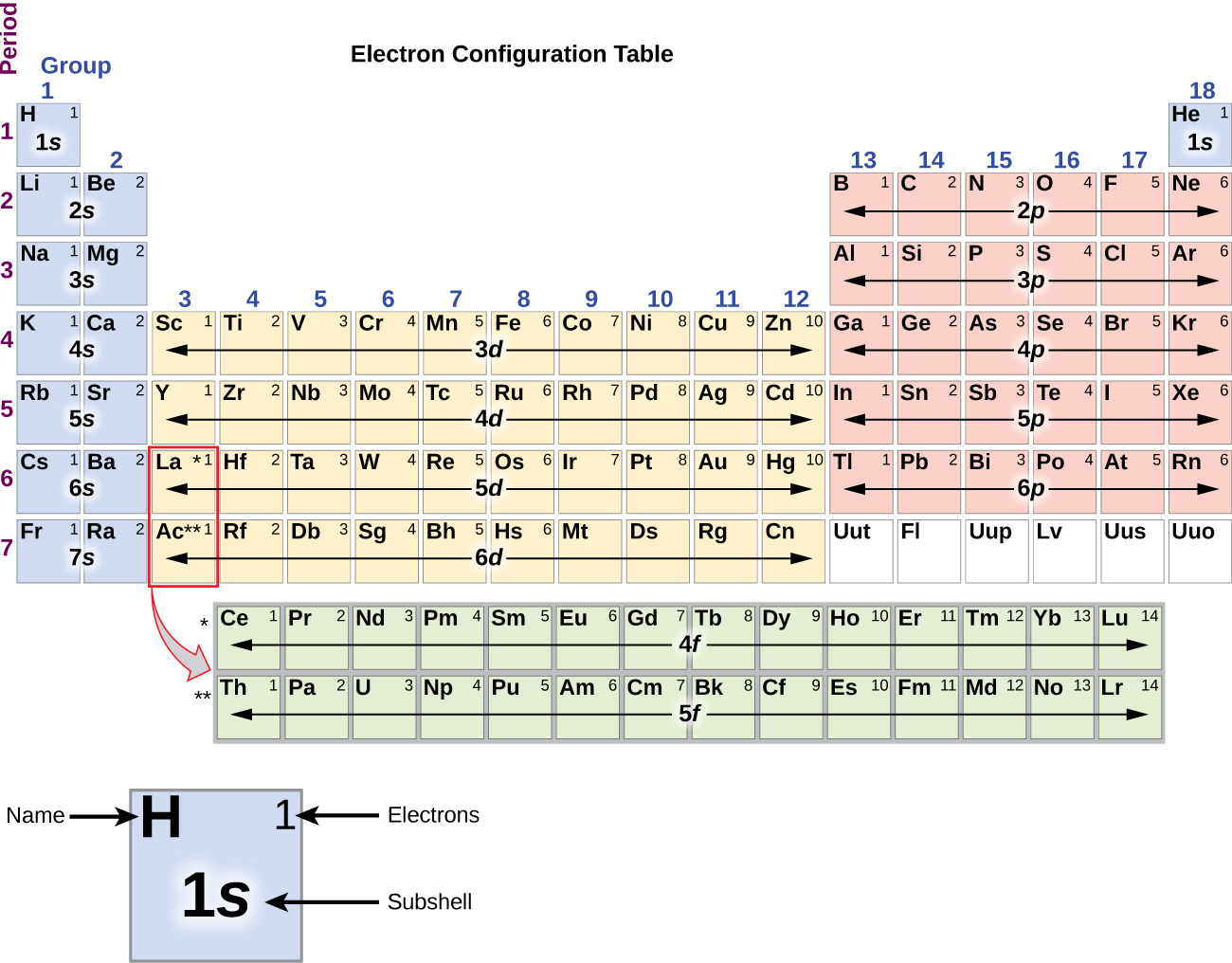
8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts

Solved 6 Order The Orbitals For A Multielectron Atom In Chegg Com
1 4 Electron Configuration And Orbital Diagrams Chemistry Libretexts
Scholarscompass Vcu Edu Cgi Viewcontent Cgi Article 1217 Context Phys Pubs

Atomic Orbital Filling Order And Electron Configurations Ap Chemistry Ppt Download
New Pt Notation

High School Chemistry Writing Electron Configurations Wikibooks Open Books For An Open World

Aufbau Principle With Exceptions Chemistrygod
Many Electron Atoms The Electronic Basis Of The Periodic Table
Structure Reactivity Atoms

Chemistry The Central Science Chapter 6 Section 9
Q Tbn 3aand9gcq6pcocjmyoorqurs8vizjbtxloj2nxidvntygwlup0ifa9h0wg Usqp Cau

Quantum Numbers

Illustration Of The Orbital Interactions That Lead To Lone Pair Download Scientific Diagram
Science Education Principles Of Distributing Electrons
Solved Part A 5s And 6s Orbitals Check All That Apply T Chegg Com

List The Sequence In Which The Following Orbitals Fill Up 1s 2s 3s 4s 5s 6s 7s 2p 3p 4p 5p 6p 7p 3d 4d 5d 6d 4f 5f Study Com

The Aufbau Principle And Hund S Rule Protocol

Shape Of The 6s Atomic Orbital On White Background Stock Photo Picture And Royalty Free Image Image
Structure Reactivity Atoms
Building Up The Periodic Table

Aufbau Principle Wikipedia

In The Sixth Period Of The Extended Form Of Periodic Table The Orbitals Are Filled As

The Orbital Concept And Hydrogenic Atomic Orbitals Youtube
Atomic Orbital Wikiwand
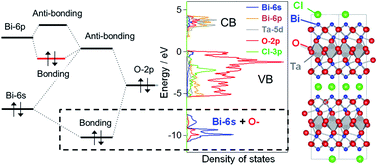
Strong Hybridization Between Bi 6s And O 2p Orbitals In Sillen Aurivillius Perovskite Bi4mo8x M Nb Ta X Cl Br Visible Light Photocatalysts Enabling Stable Water Oxidation Journal Of Materials Chemistry A

Quantum Numbers Atomic Orbitals And Electron Configurations

Quantum Mechanical Model Electron Configurations Ppt Download

Bohr Transitions For Electron Orbitals Iphone 5s Case For Sale By Science Photo Library
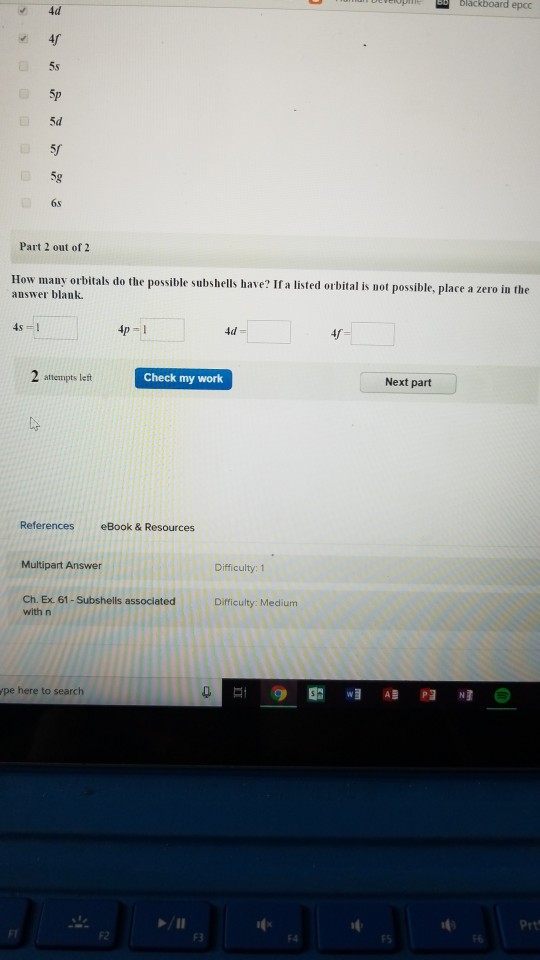
Solved Blackboard 40 5s 5d 50 6s Part 2 Out Of 2 How Chegg Com
6p Elements In Periodic Table Structure Of The Periodic Table

Why Is Mercury A Liquid At Stp

List These Electron Subshells In Order Of Increasing Energy 3d 4d 5s 4p Note For Advanced Homeworklib
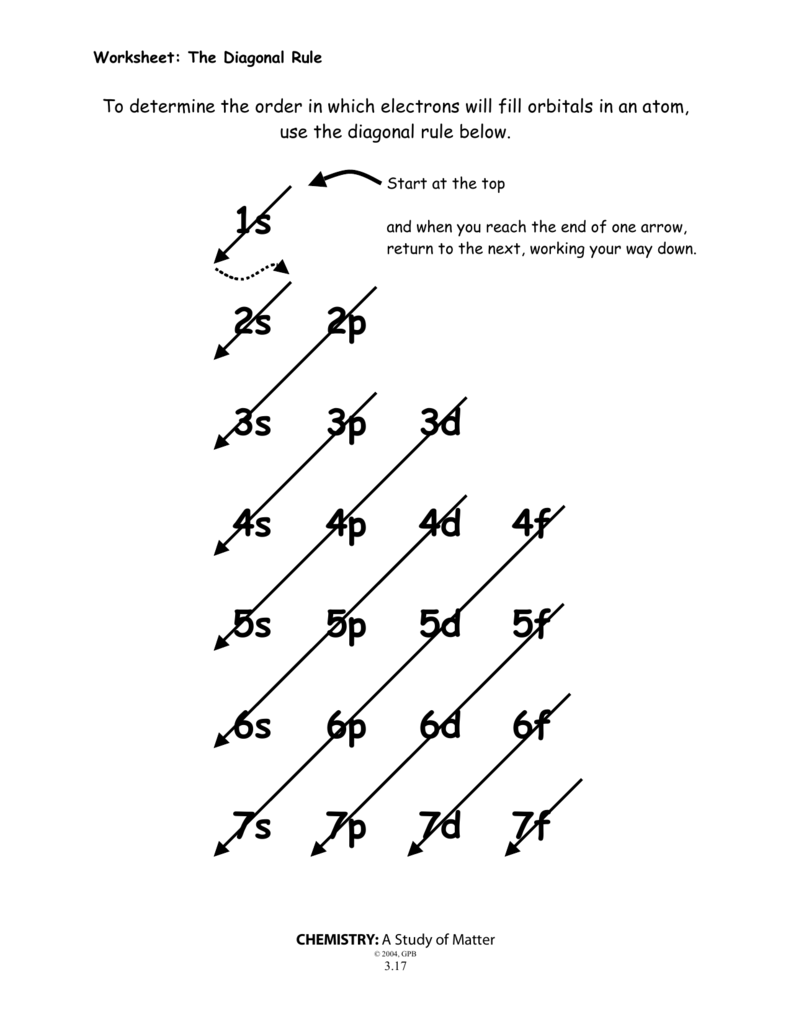
1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 6f 7s 7p 7d 7f

01 The Increasing Order Of Energy Of Orbitals Is 1 4f 6p 6d 2 4d 5s 5p In 3 5d 41 6s 4 6p 5d 6d

What Is The Noble Gas Shorthand Electron C Clutch Prep
Electron Configurations

Aufbau Principle
Electron Configuration Wikipedia

Writers Outlet Creative Writing Library Share Connect

Electron Configurations

Energy Levels Of The S And D Orbitals In A Single Copper Silver And Download Scientific Diagram

A Molecular Orbital Plots Of Ir 5d Orbitals In R 3 Irf 8 B Download Scientific Diagram
Aufbau Principle
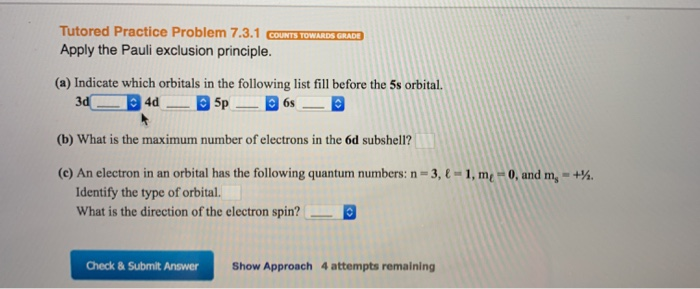
Solved Tutored Practice Problem 7 3 1 Counts Towards Grad Chegg Com
What Is The Reason Why Only The P Orbital Form Pi Bonds Quora

Are Orbitals Always Filled In From Closest To Nucleus To Farthest Away Chemistry Stack Exchange

Figure 2 From The Renaissance Of Non Aqueous Uranium Chemistry Semantic Scholar
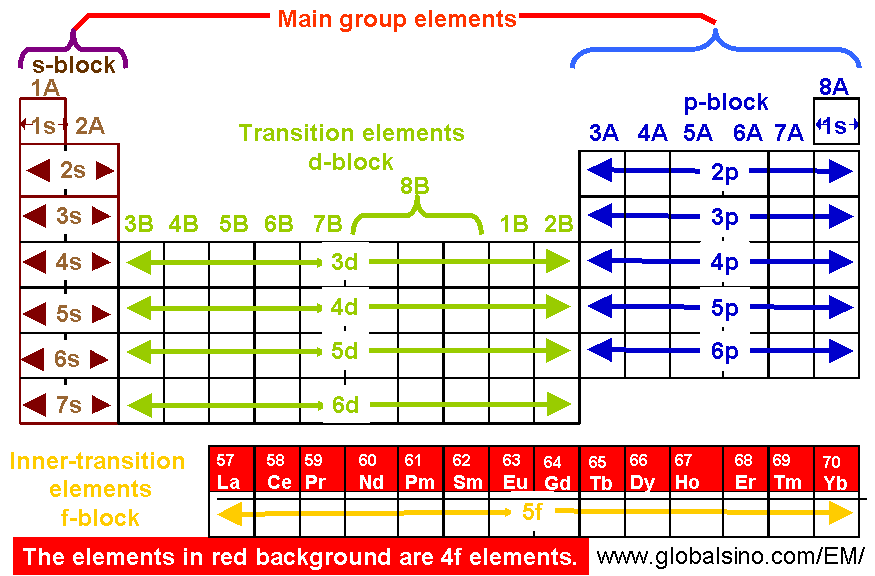
4f Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book
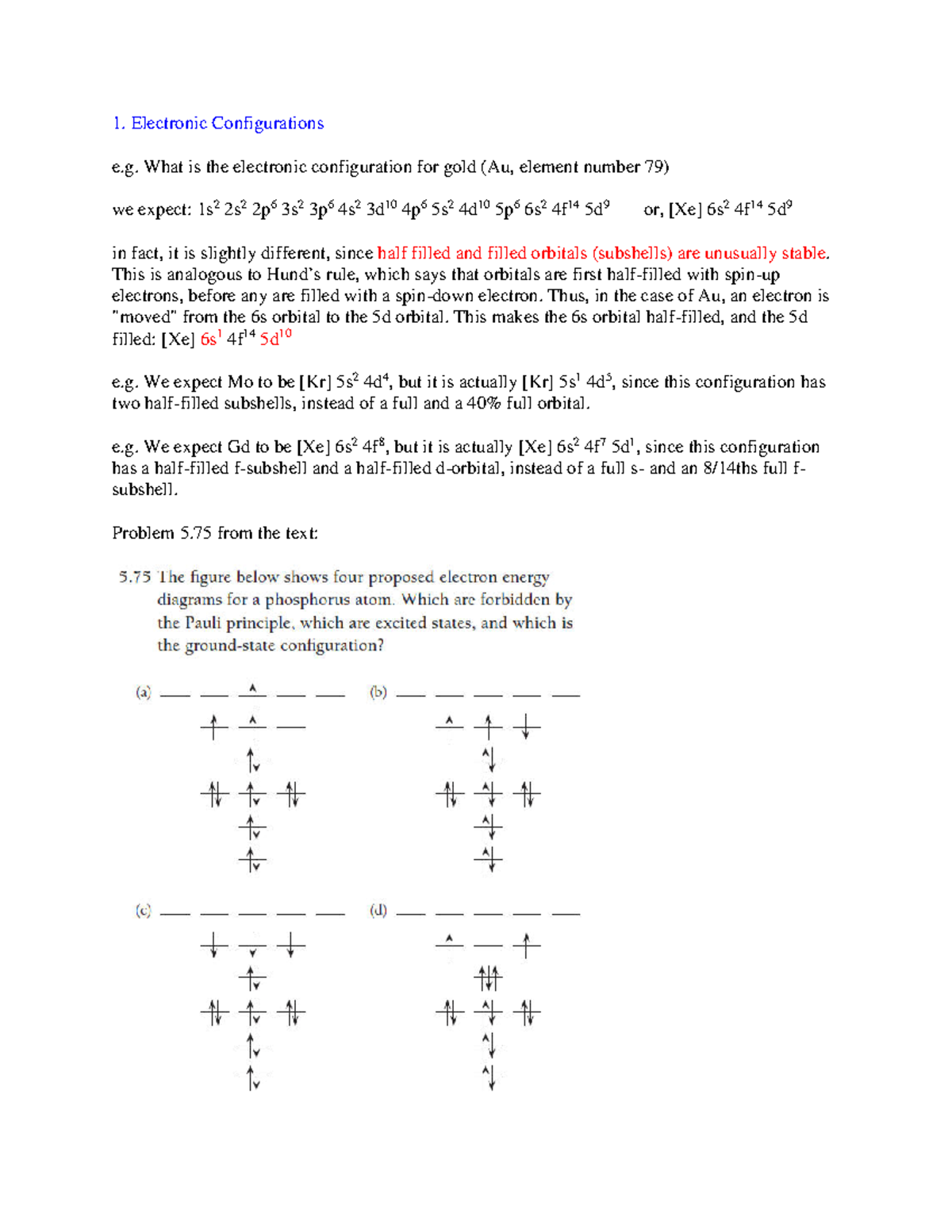
5 Atomic Energies And Periodicity Chem 1001 Carleton Studocu
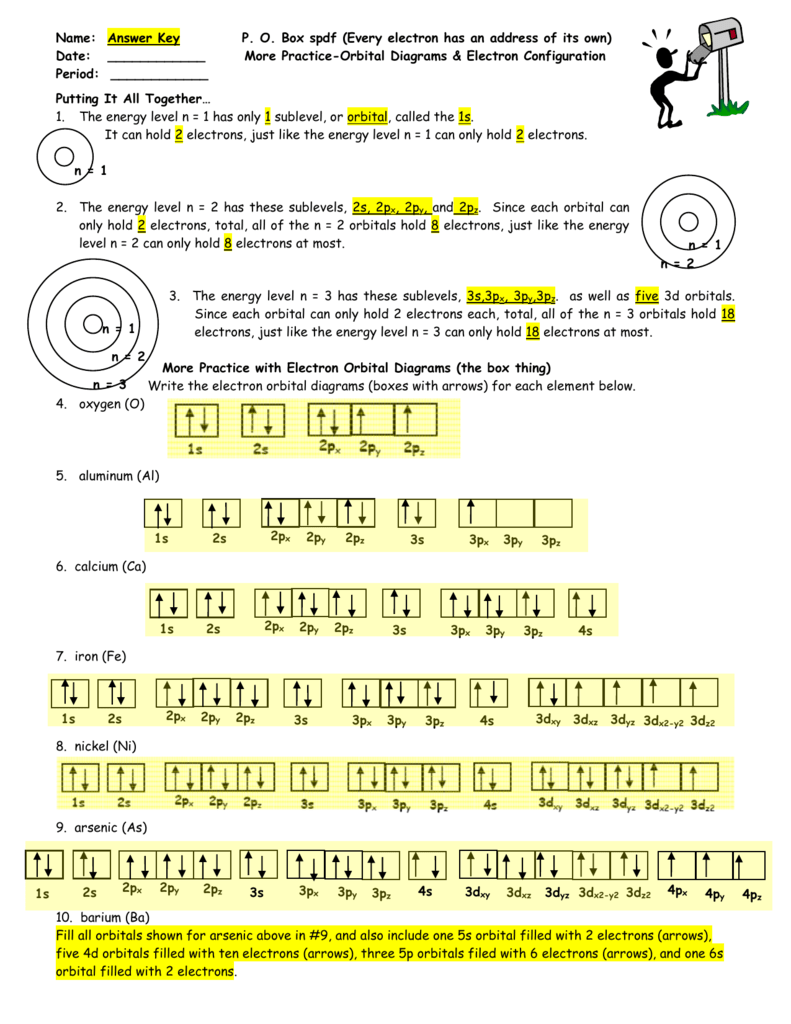
Po Box Spdf Worksheet Answer Key
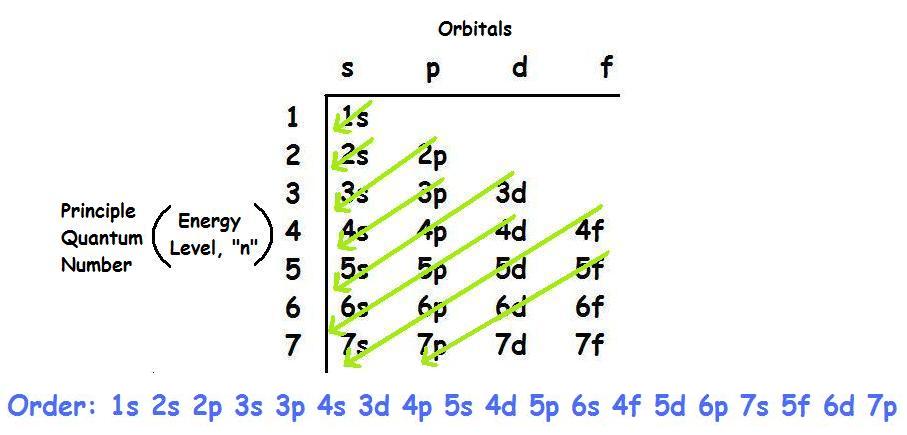
What Is The Ground State Electron Configuration Of The Element Kr Socratic
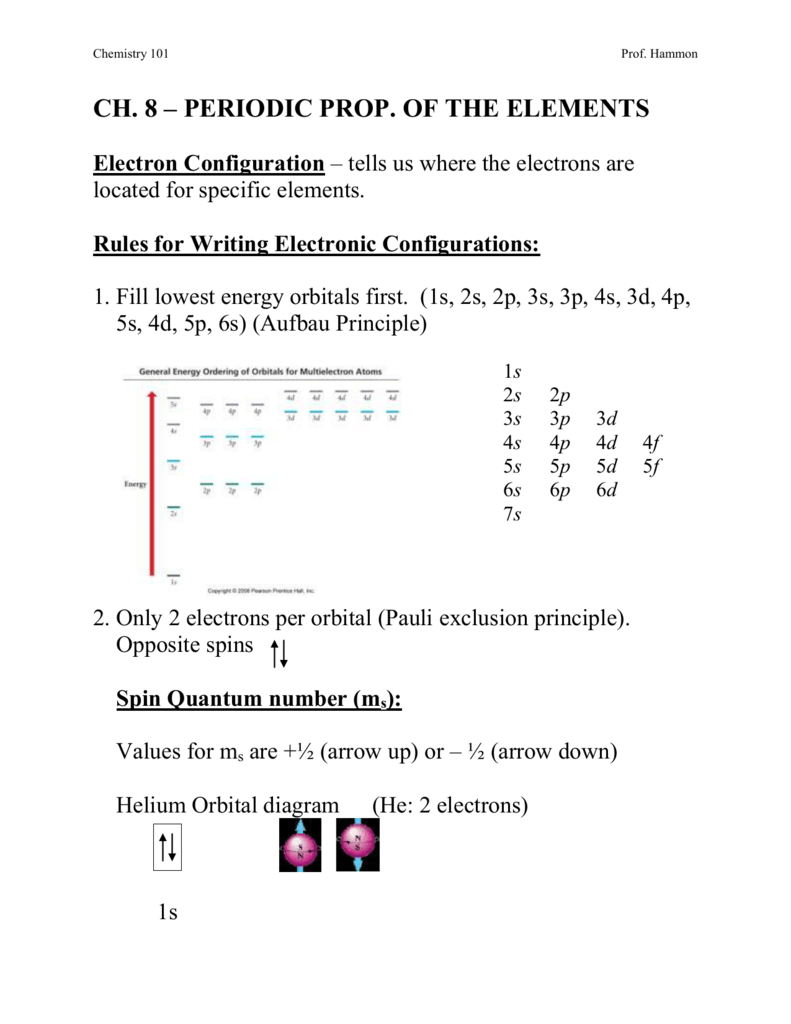
101ch8

Energy Levels Orbitals Ppt Download
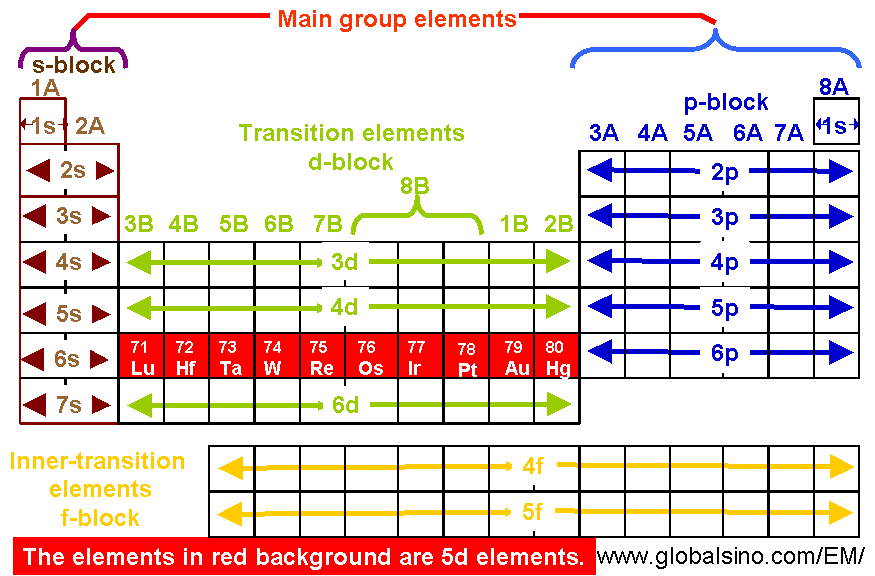
5d Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book
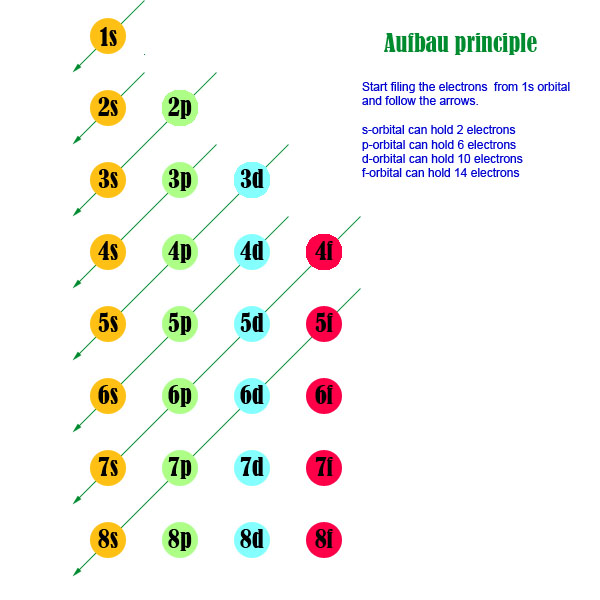
Which Sublevel Is Filled After The 5s Sub Level Socratic

Atomic Orbital Wikipedia

Electron Configuration Ppt Download
Q Tbn 3aand9gcsfibjyyyxfonwfamefq8 Vqv8ugu2ydlp4yb Nuyulhve5woqc Usqp Cau
Electronic Structure Of Atoms Electron Configurations Chemistry Atoms First

Solved Arrange The Orbitals For A Many Electron Atom In E Chegg Com
1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f 6s 6p 6d 7s 7p

Chapter 8 Handout 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p Electron Configuration Atomic Orbital

Periodic Table The Basis Of The Periodic System Britannica

The Atom 4 Hydrogenic Orbital Shapes Energies Filling Youtube

Chemquest 18 Electron Configuration Atomic Orbital
Q Tbn 3aand9gcrjaqjlb8lpy2zmnxb7djnungtx4z3mbsdjg7v7iofb5bmcmoq Usqp Cau

A Electron Configuration And The Periodic Table

Clarifying Electron Configurations Chemical Education Xchange
Summary Of Electron Configurations
1
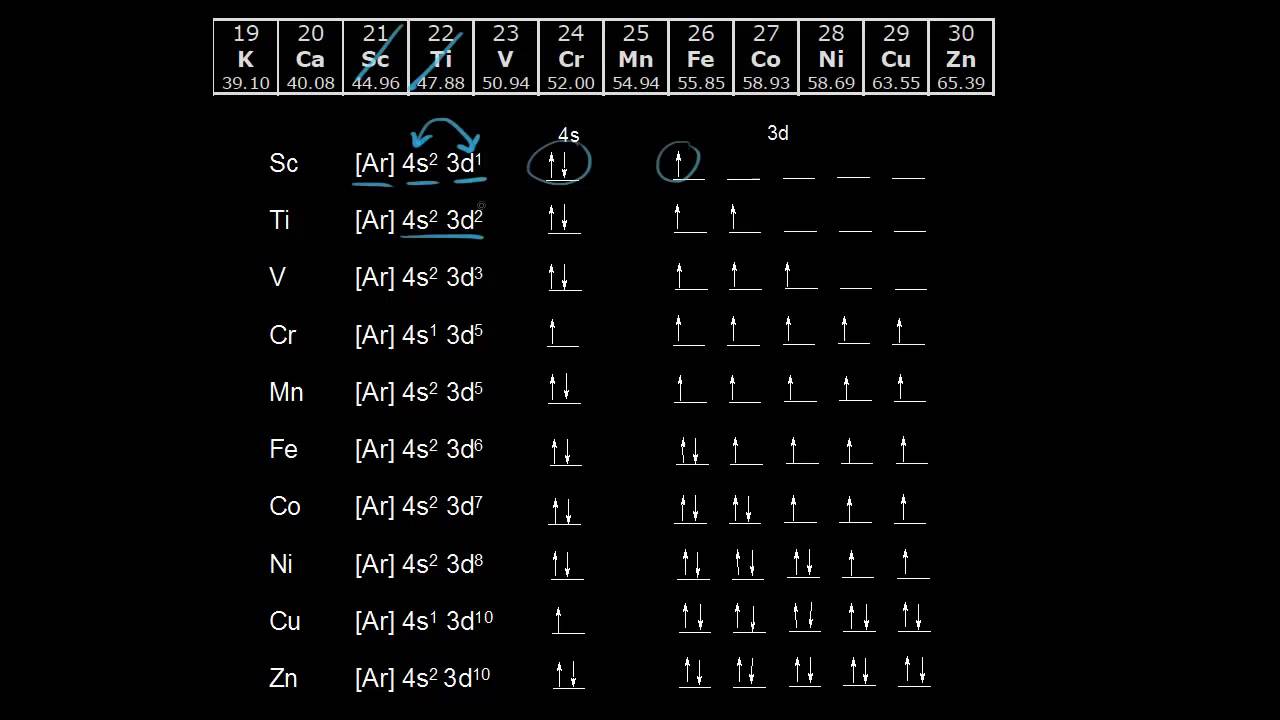
Electron Configurations Of The 3d Transition Metals Video Khan Academy
What Is The Electron Configuration Of Elements Quora
Structural Characterization Of Small Xe Clusters Using Their 5s Correlation Satellite Electron Spectrum Physical Chemistry Chemical Physics Rsc Publishing
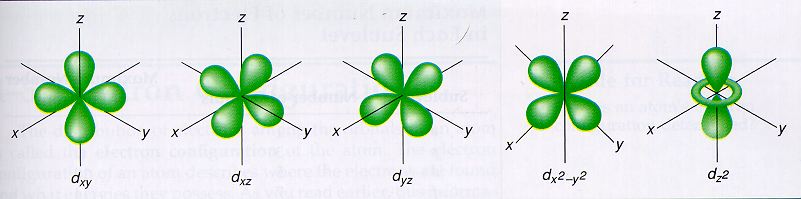
2a3 Orbitals

High School Chemistry Writing Electron Configurations Wikibooks Open Books For An Open World
New Pt Notation
A Electron Configuration And The Periodic Table

Aufbau Principle Ck 12 Foundation

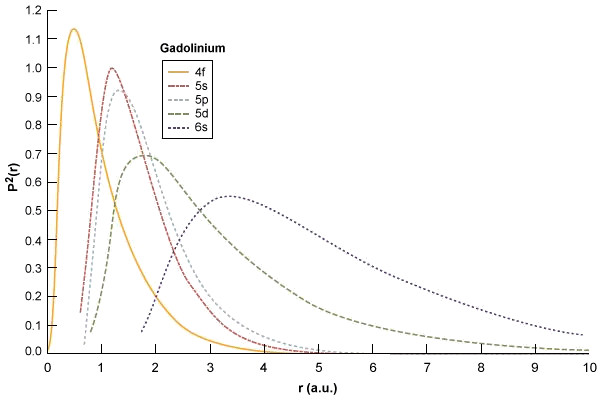
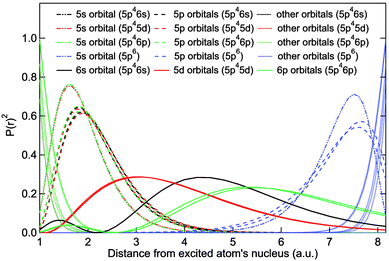
Square Of The Radial Wavefunctions For The 4f 5s 5p And 6s Energy Download Scientific Diagram

Electron Configurations
2

Solved What Is The Order For Filling The Following Subshe Chegg Com

Which Of The Following Electronic Transitions Is Consistent With A Decrease In Energy 5s Subshell Homeworklib

Square Of The Radial Wavefunctions For The 4f 5s 5p And 6s Energy Download Scientific Diagram

Shape Of The 6s Atomic Orbital On White Background Available Stock Photo Picture And Royalty Free Image Image 5717
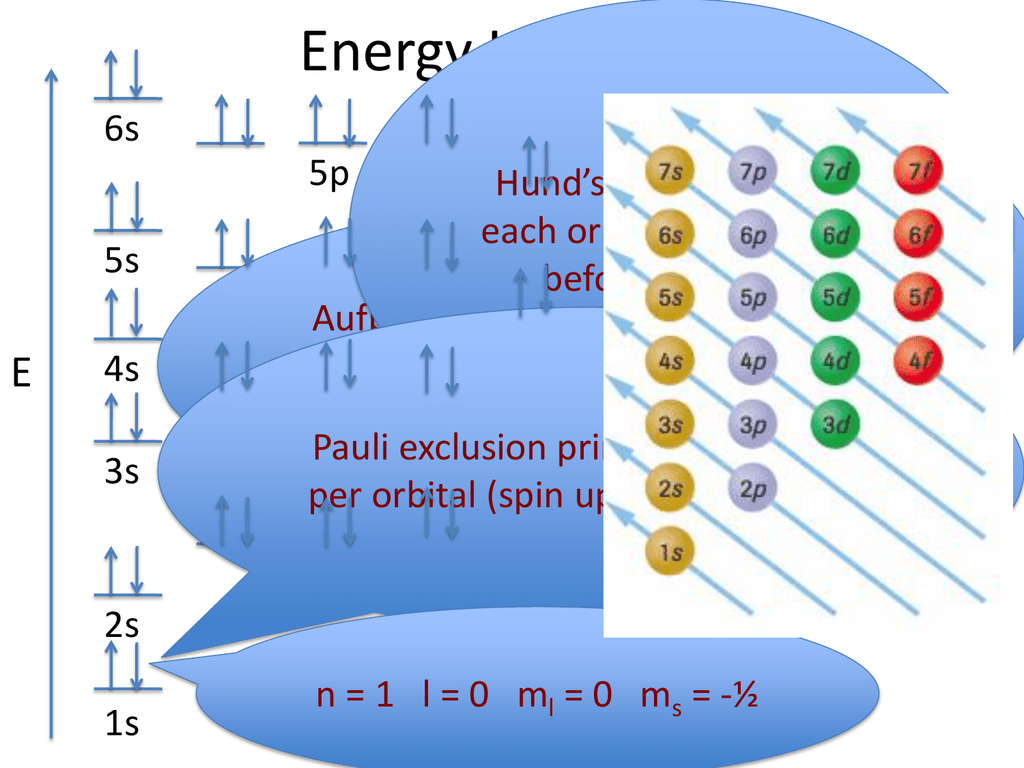
Electron Configuration
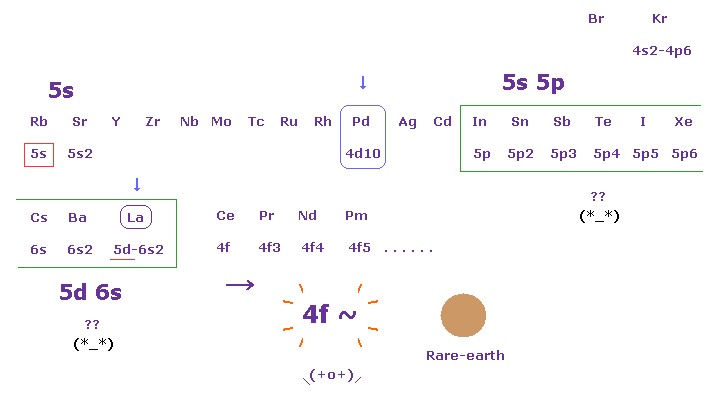
Ls Coupling And Magnetization Of 4f Rare Earth 3d Metals

Solved Identify What Is The Same For A D 1 They Have Th Chegg Com
A Electron Configuration And The Periodic Table

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
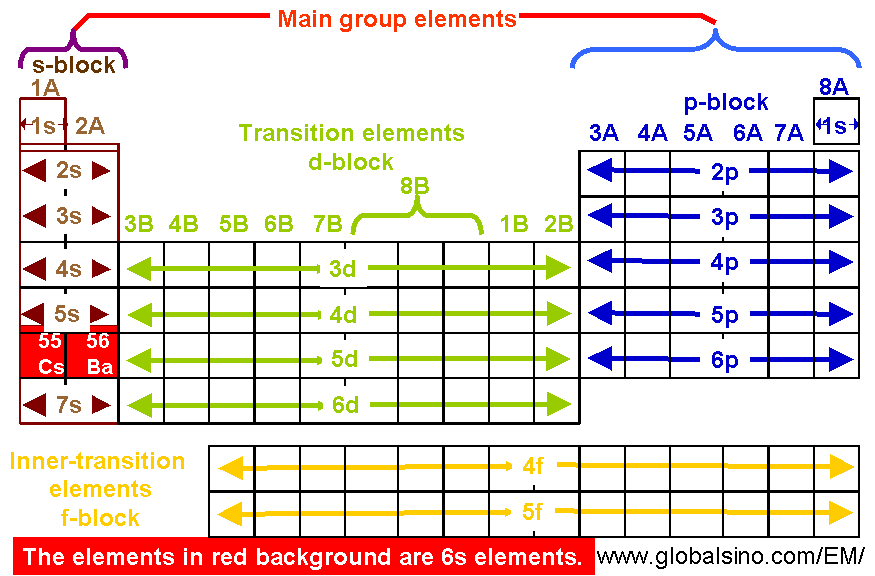
6s Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book



