6s Orbital Electrons
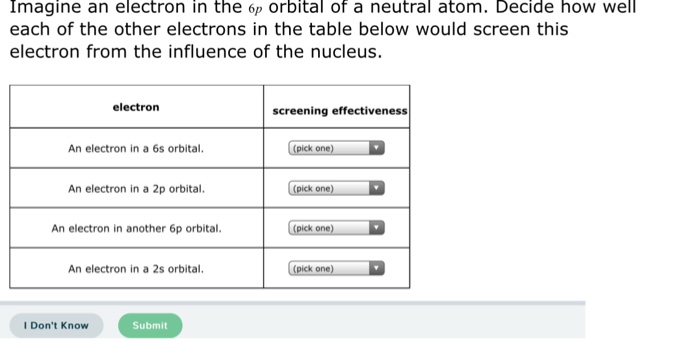
Solved Imagine An Electron In The 6p Orbital Of A Neutral Chegg Com

What Is The Noble Gas Shorthand Electron C Clutch Prep
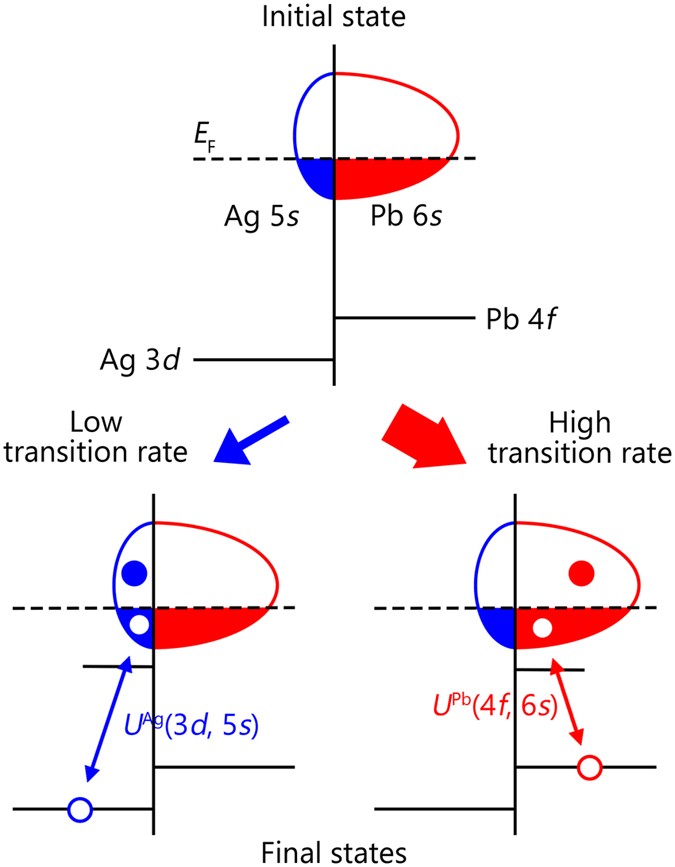
Element Specific Orbital Character In A Nearly Free Electron Superconductor Ag 5 Pb 2 O 6 Revealed By Core Level Photoemission Scientific Reports

Chapter 6 Section 8

What Is The Total Number Of Electrons Possible In The 6s Orbital

Energy Levels Of The S And D Orbitals In A Single Copper Silver And Download Scientific Diagram
Due to the Heisenberg uncertainty principle, it is not possible to know both the position and the momentum of the ele.

6s orbital electrons. For example, s orbital holds 2 electrons, while p holds 6. This causes mercury to behave like a noble gas. The electrons in n=4 and lower shield by 1.00.
Do a 4s and a 4p subshell have the same energy?. L for p orbital is 1. The Principal Quantum Number (\(n\)) The principal quantum number, \(n\), designates the principal electron shell.
How many unshared pairs of electrons are in this orbital diagram. Electrons are speed throughout the atom on different levels and subshells. In all the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital.
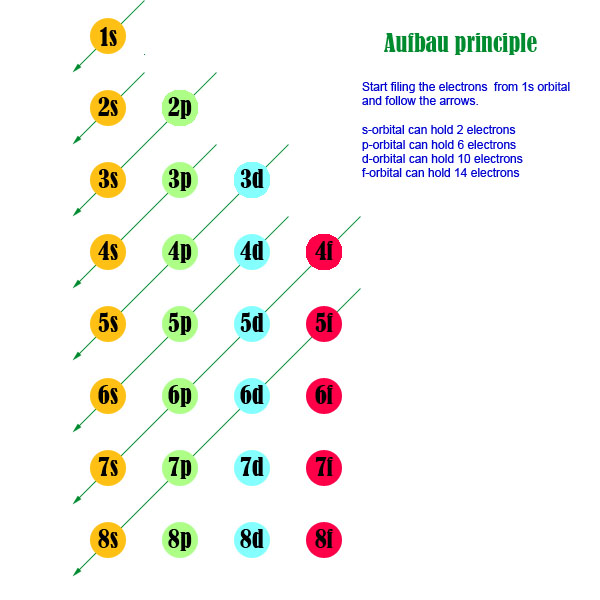
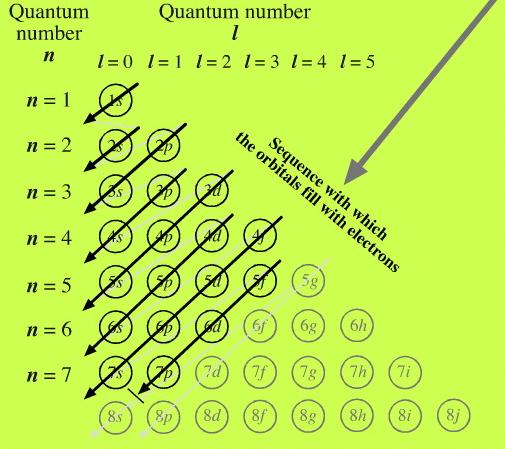
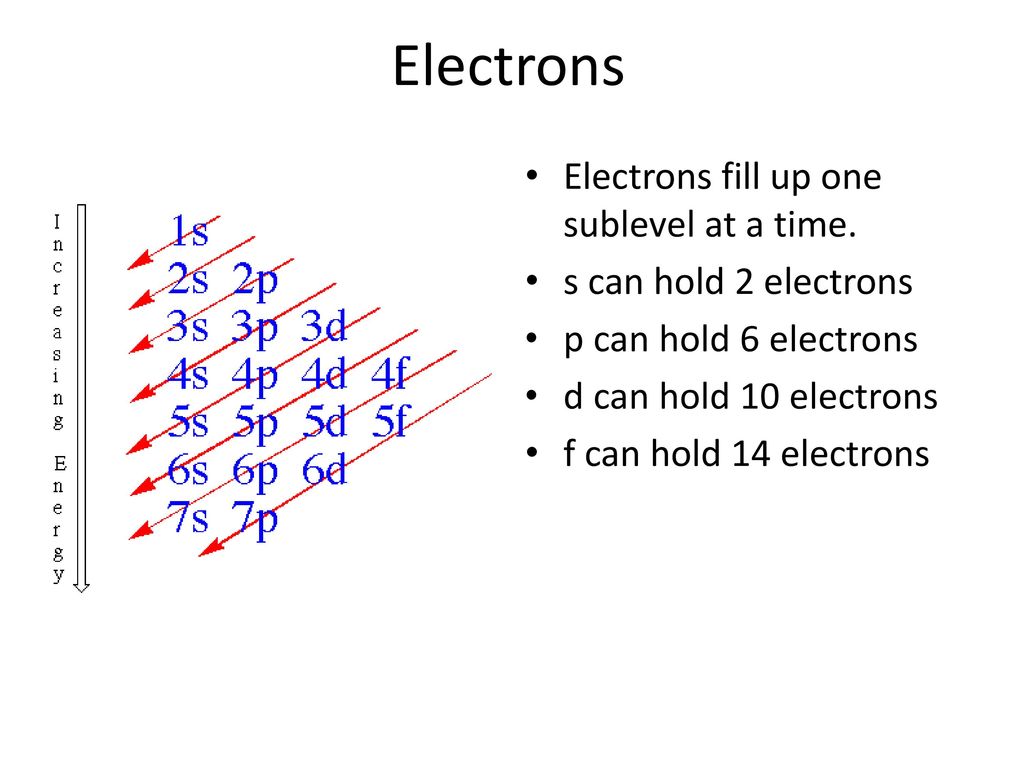
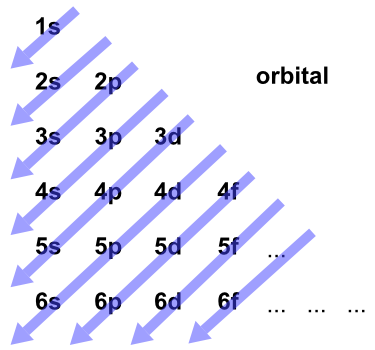
These general rules result in the following orbital filling order:. S - orbital (maximum electrons = 2) 2. The distribution of electrons among the orbitals of an atom is called the electron configuration.The electrons are filled in according to a scheme known as the Aufbau principle ("building-up"), which corresponds (for the most part) to increasing energy of the subshells:.
The secondary quantum number l specifies the shape of the orbital. For any atom there is just one 6s orbital. Because of this effect, the 6s orbital is lower in energy relative to the 5d orbital than the 5s is relative to 4d or the 4s to 3d.
The p sublevel has three orbitals and can contain 6 electrons. Then comes 4d, 5p, and 6s. Imagine an electron in the 6p orbital of a neutral atom.
How many electrons in 6s subshell?. Next comes 3d, 4p, and 5s. They are in the same energy level.
10 electrons can be present. For 5d orbital, (n + l) = 5 + 2 = 7. The electron configuration for calcium is:.
To determine the orbital from a given pair which has higher energy in a many electron atom. There are 4 types of orbital:. S, p, d, f.
For 6s orbital, (n + l) = 6 + 0 = 6. However, the electron can exist in spin up (m s = +1/2) or with spin down (m s = -1/2) configurations. An orbital holds 2 electrons.
Furthermore, after every noble gas element, the ionization energy drastically drops. Last comes 5f, 6d, and 7p. This view is getting some support by the fact that the 6s orbital expands when proceeding beyond mercury, where the 6p orbitals are filled in.
“Lazy” Electrons are located in the orbital with lowest energy level possible. Consider the outer electrons first. How many unshared pairs of electrons are in this orbital diagram?.
“Unique” No two electrons can share the same position and spin. A 6s orbital fills before a 4f and 5d because of the Aufbau Principle.) Pauli Exclusion Principle - An atomic orbital can describe at most two electrons. For 4f orbital, (n + l) = 4 + 3 = 7.
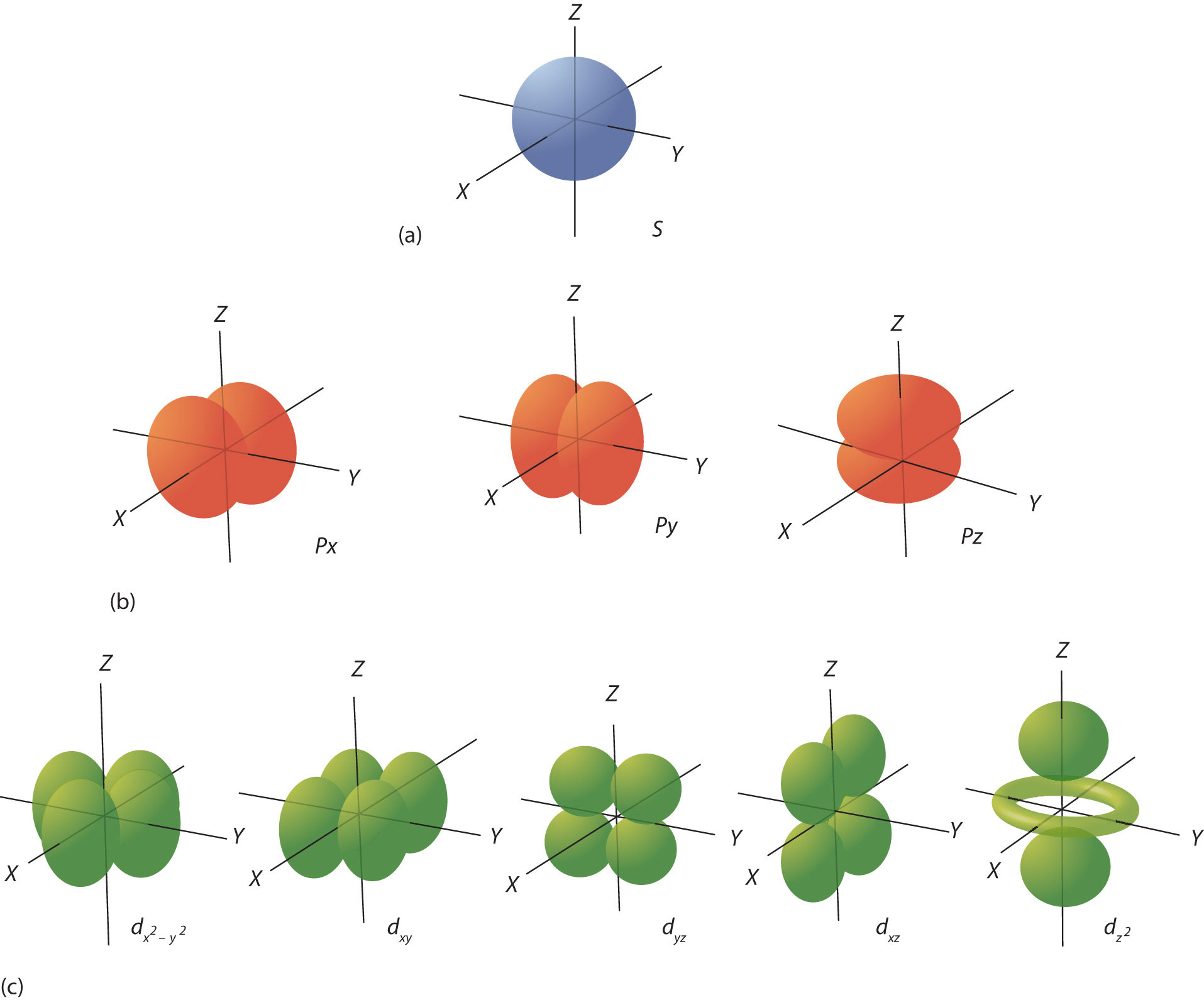
• A letter indicates the type of orbital;. Each orbital is spherical, with the nucleus at the center of the sphere. So, the most frequently used names for the s orbitals are 1s, 2s, 3s, 4s, 5s, 6s and 7s.
Term and Answer Keys. The electrons have their own orbital filling mechanism. The order of the electron orbital energy levels, starting from least to greatest, is as follows:.
The energy shell below that is n = 5. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. L for d orbital is 2.
So 6s = 6+0 =6. The 1s orbital is the smallest, and the 7s orbital is the largest. The p sublevel has 3 orbitals, so max.
An orbital can contain two electrons only if the electrons have opposite Which model allows a student to determine the total number of electrons in an atom and the electrons within Arsenic does not have any valence electrons in the 3d orbital because A - its 3d orbital is completely. Electrons add in energy order (Aufbau Principle) not energy level order. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 According to this configuration, how many electrons are found on the valence.
They have the same shape. 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f. The main difference between s orbitals is in the size.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6. 2) Do 5s and 6s orbitals have the same maximum number of electrons?- Yes, they are both s, so can hold 2 elec. Is a 1s orbital the same energy as a 7s orbital?.
Because n describes the most probable distance of the electrons from the nucleus, the larger the number n is, the farther the electron is from the nucleus, the larger the size of the orbital, and the larger the atom is.n can be any positive integer starting at 1, as \(n=1. The s sublevel has one orbital and can contain 2 electrons. Electrons that are on the same orbital have different spins, clockwise and counter clockwise.
D - orbital (maximum electrons = 10). 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. How many electrons in a 6p orbital?.
Thus, the energy gained by not shoving two electrons into the 6s orbital, plus that from a half-filled 5d subshell, is not enough to compensate for promoting an electron from 6s to 5d. Which type of orbitals are shaped like a propeller?. In all other respects, the 4s electrons are always the electrons you need to think about first.
For a 6s orbital, n = 6. The origin of the spherical nodes becomes clearer upon examining the wave equation for this orbital. An electron pair means electrons that stay together in a sub-shell or orbital.
Furthermore, how do you know which orbital is higher in energy?. Therefore, n = 3 and, for a p-type orbital, l = 1. Maximum number of electron in an energy level (2n 2) Principal Energy Level (n) sublevels:.
The superscript is the number of electrons in the level. The element is helium. The m l value could be –1, 0, or +1.
The image on the left is deceptively simple as the interesting features are buried within the orbital. The orbit in which the electrons will be added depends upon its energy level. However, in Nb as the new entrant electron is bound to enter into the vacant 5d orbital it helps in pulling 1 of the 2 electrons of 6s orbital into the 5d orbital due to sheer electronic number superiority.
(click to see image?. This means that the s orbital can contain up to two electrons, the p orbital can contain up to six electrons, the d orbital can contain up to 10 electrons, and the f orbital can contain up to 14 electrons. The s sublevel has only one orbital, so max.
Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s.The filling order is based on observed experimental results, and has been confirmed by theoretical calculations. To be more explicit, to the stronger shielding of the nuclear charge by p electrons, as compared to d and f electrons. Barium is in group 2, which is in the s block, and it is in period 6, so the 6s orbital will be the orbital that holds its valence electrons.
P - orbital (maximum electrons = 6) 3. There are 16 electrons in n = 5;. L is azimuthal quantum no.
L for s orbital is 0. 6s 4f 5d 6p. You could be either asking for this OR s orbital contains maximum of 2 electrons (has 1 orbitals) p orbital contains maximum of 6 electrons (has 3.
The 15 electrons of the phosphorus atom will fill up to the 3p orbital, which will contain three electrons:. For an atom with 118 electrons, the electron orbital configuration would be:. In other words, when we.
The two electrons in the same orbital are closer together on average than two electrons in different orbitals, so that they shield each other more effectively and it is easier to remove one, resulting in a lower ionization energy. Decide how well each of the other electrons in the table below would screen this electron from the influence of the nucleus. The last electron added is a 3p electron.
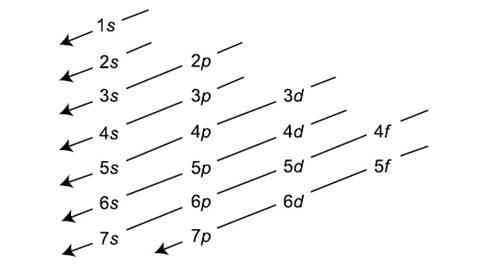
This change of the trend has to be attributed exclusively to orbital effects;. And the 4 sub-levels have seven orbitals, and they can hold max 14 electrons. Electrons fill orbitals in order of increasing (n + ) and when two or more subshells have the same (n + ) value, electrons fill the orbital with the lower n value.
According to the Bohr Bory principle E= n+l Where E is the energy of the orbitals,n is the principal quantum number and l is the azimuthal quantum number Now according to the Aufbau's principle electrons are filled in the orbitals with their incre. The number of electrons contained in each subshell is stated explicitly. It suggests that electrons will always fill an s orbital and then will proceed with p orbital.
Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. 1s3- There is only one s orbital per energy level, and each orbital can hold a maximum of two electrons, so there can not be 3s electrons at any energy level. How many unshared pairs of electrons are in this orbital diagram?.
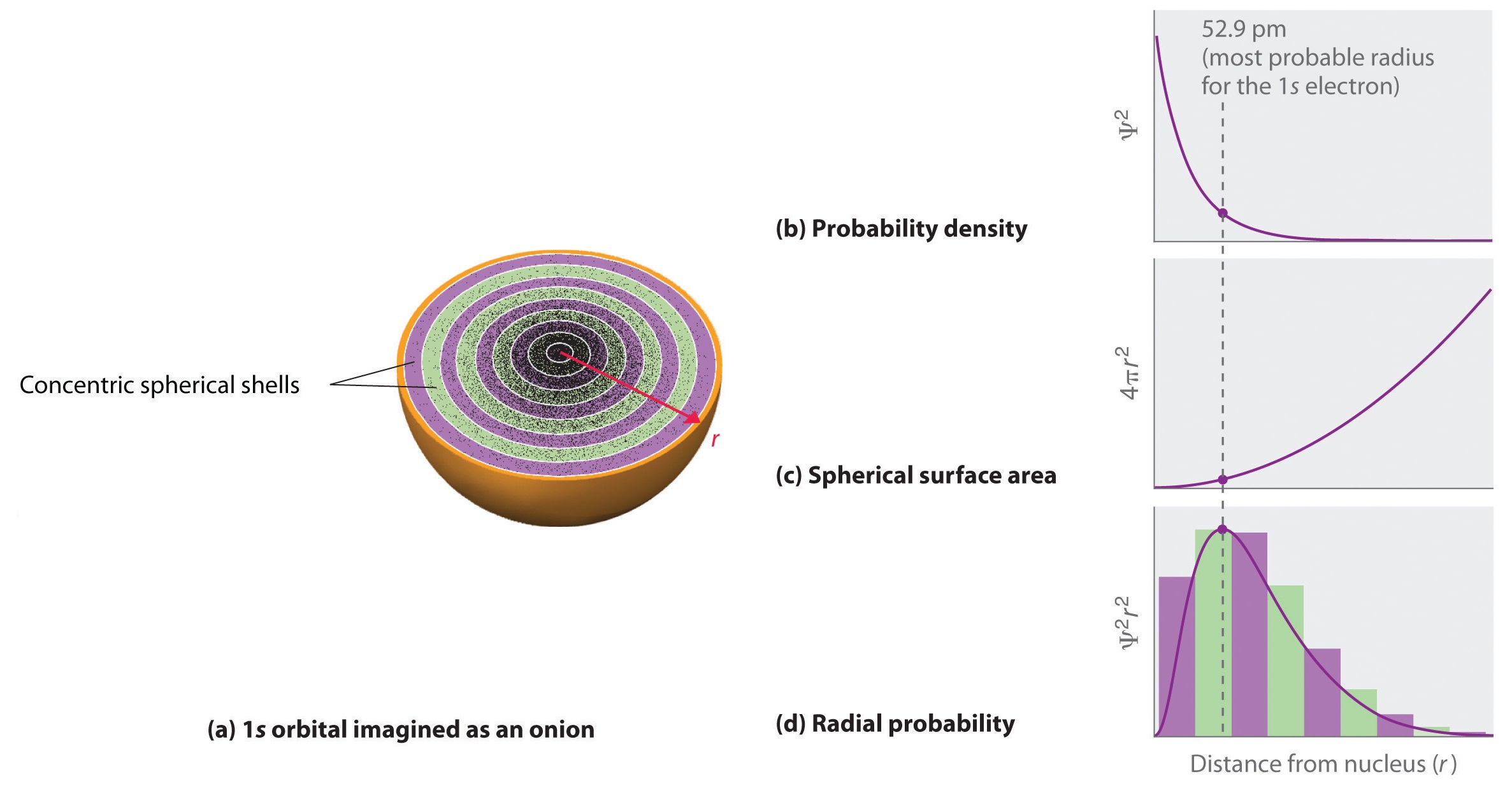
Each orbital contains maximum or 2 electrons. For example, the electron configuration of beryllium, with an atomic (and electron) number of 4, is 1s 2 2s 2 or He2s 2. That on the right is sliced in half to show that there are five spherical nodes in the 6s orbital.
Which type of orbitals are shaped like a sphere?. (pick one) 1 (most effective) alo An electron in a 4f orbital. Electron screening effectiveness ha An electron in a 6s orbital.
Since electrons all have the same charge, they stay as far away as possible because of repulsionAt the lowest energy level, the one closest to the atomic center, there is a. For unpaired electrons. The two electrons in its 6s orbital are happily paired up with each other and are reluctant to be shared among neighbouring atoms.
Atomic orbitals are represented using a box. An electron always enters an orbital having lowest energy. The d sublevel has five orbitals and can contain 10 electrons.
Then electrons (arrows) are added one at a time into each type of orbital. And it is calculated by n + l. L = 0 specifies a spherical orbital.
After filling 6s, electrons would fill. Since we know that energy of an electron is determined by its principal quantum number ‘n’ denotes the principal energy level of an electron. Cerium Gadolinium Lutetium in Periodic Table.
1s electron in Os. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6. How To write an electron configuration:.
So lower the n +l value of an orbitalelectro s will be filled in tht orbit first. Since electrons all have the same charge, they stay as far away as possible because of repulsion. The maximum number of electrons is the same.
It is the only metal that is monatomic in the gas phase, not forming diatomic molecules like its cousins. Just as in a seesaw similarly in Zr equal number of 2 electrons are present each for 6s and 5d orbitals which balances each others forces;. Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.
“Share” When orbitals have the same energy level, each orbital gets one electron before any orbital gets two. Ls2 means that there are two electrons in the ‘s’ orbital of the first energy level. F-block chemical elements like Cerium, Gadolinium, and Lutetium in the periodic table contain one electron in 5d orbital with atomic number 58, 64, and 87.
Also, the s orbitals occur singly. • Determine the total number of electrons to be represented. (1s) 2 (2s2p) 8 (3s3p) 8 (3d) 10 (4s4p) 8 (4d) 10 (4f) 14 (5s5p) 8 (5d) 6 (5f) 0 (6s6p) 2.
2 electrons can be present. N is principal quantum no. How many orbitals in n = 3 shell?.
The reversed order of the 3d and 4s orbitals only seems to apply to building the atom up in the first place. Check all that apply. **Remember the super script is the number of electrons, so 6s2 (2 electrons (arrows) in the s), 4f 14 (14 electrons (arrows) in the f), 5d10 (10 electrons (arrows) in the d), 6p3 (3 electrons.
Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m dubious – discuss, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number).Each such orbital can be occupied by a maximum of two electrons, each with its own spin quantum number s. The d sublevel has 5 orbitals, so max. For beryllium, there are two electrons in the 1s orbital and 2 electrons in the 2s orbital.
3) Are 5s and 6s orbitals in the same energy level - No, 5s is of higher energy 4) Do. The electron of interest is in the 6s orbital. Therefore, the outer orbital contains 6s-electrons and the inner energy orbital contains f-electrons.
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. After filling 6s, electrons would fill. The 5p orbitals fill immediately after the 4d orbitals and immediately before the 6s because:-observed experimental results-theoretical calculations.
The total number of electrons in a given atom is equal to its atomic number. After that comes 4f, 5d, 6p, and 7s. While still spherical, higher s-orbitals (such.
As far as I know, and someone with a better way to explain and understand this may well come along and say more or contradict me:. How many unshared pairs of electrons are in this orbital diagram?. • A superscript indicates the number of electrons in the orbital.
(Which ones apply to the 5s and 6s orbitals:. If an orbital contains only one electrons, it would be written like this:. For an s orbital, l = 0.
The order of the electron orbital energy levels, starting from least to greatest, is as follows:. The three p orbitals are degenerate, so any of these m l values is correct. 6 electrons can be present.
The allowed values of l are 0, 1, 2, 3 … n - 1. According to quantum theory, the principal quantum number n specifies the size and energy level of the orbital.

8 4 Electronic Structure Of Atoms Chem 1114 Introduction To Chemistry

A Electron Configuration And The Periodic Table
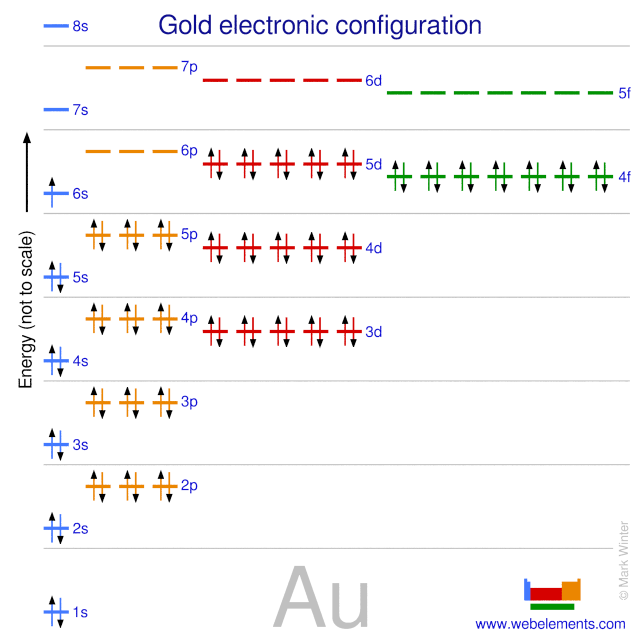
Webelements Periodic Table Gold Properties Of Free Atoms
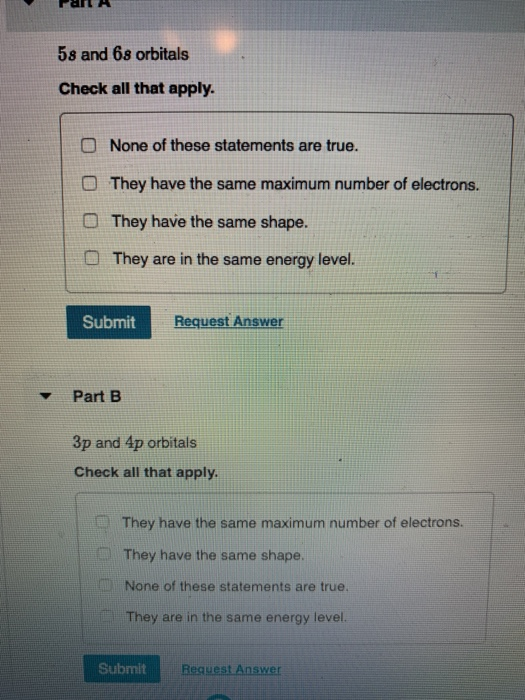
Solved 5s And 6s Orbitals Check All That Apply O None Of Chegg Com

Relativistic Effects In Your Wedding Ring Sciences In The Mural Of Life
6 6 3d Representation Of Orbitals Chemistry Libretexts
Electron Configuration Of Elements Ions

When Electrons Are Added In The 6s Orbital What Happens To The Energy Level Of The 5d Orbitals Brainly In

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

Orbital Diagrams And Electron Configurations Vocabulary 1 Electron Configuration 2 Aufbau Principle 3 Pauli Exclusion Principle 4 Electron Spin 5 Hund S Ppt Download
A Electron Configuration And The Periodic Table
Which Sublevel Is Filled After The 5s Sub Level Socratic

Electron Configuration Ppt Download

Atomic Orbital Wikipedia
The Trouble With The Aufbau Principle Feature Rsc Education

How To Find The Number Of Orbitals In An Atom Also How Many Electrons Are In Those Orbitals Quora
5d Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book

Gaussian Basis Sets For The Calculation Of Some States Of The Lanthanides
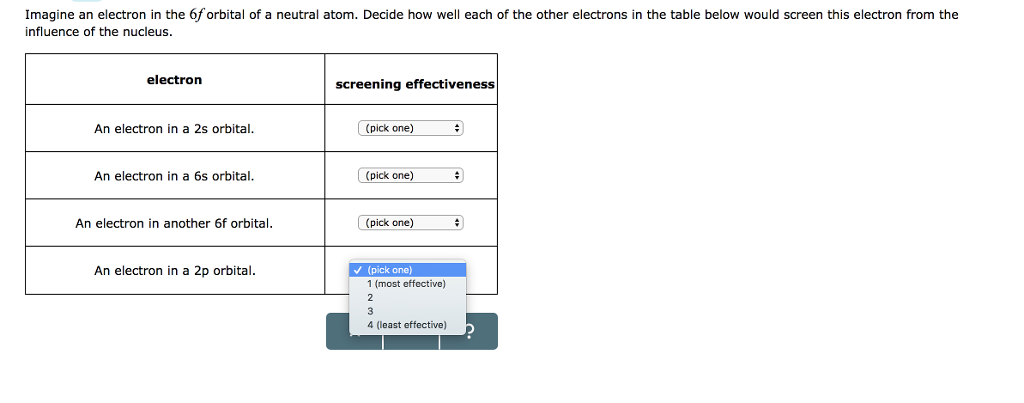
Solved Imagine An Electron In The 6f Orbital Of A Neutral Chegg Com

Electronic Structure Of Atoms Electron Configurations Chemistry Openstax Cnx
Quantum Numbers For Electrons
Science Education Principles Of Distributing Electrons

Quantum Numbers
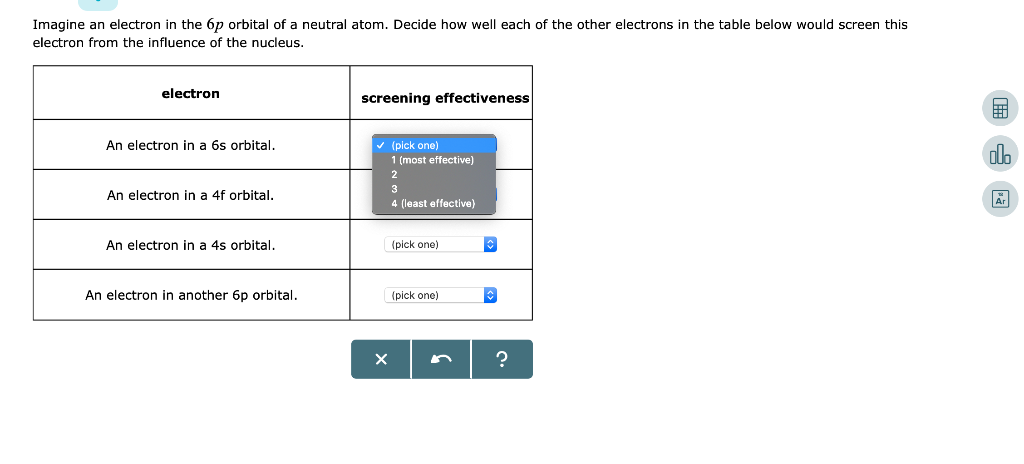
Solved Imagine An Electron In The 6p Orbital Of A Neutral Chegg Com
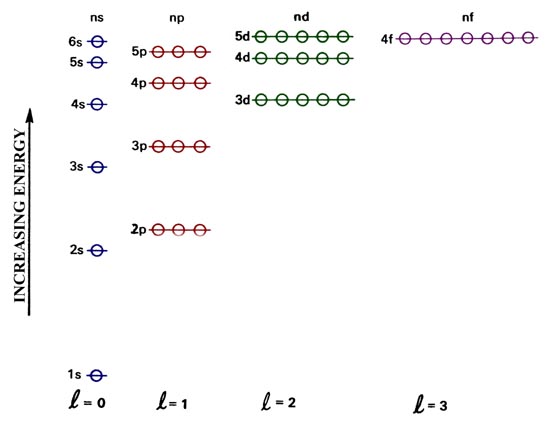
Many Electron Atoms The Electronic Basis Of The Periodic Table
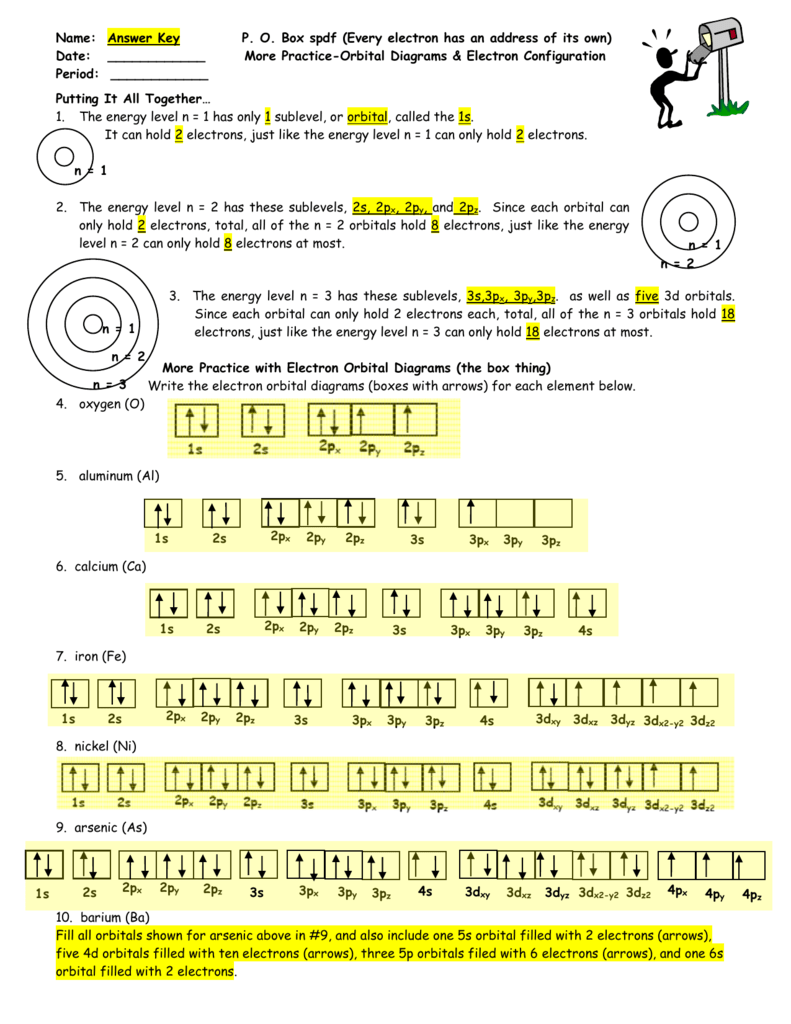
Po Box Spdf Worksheet Answer Key
Q Tbn 3aand9gcqalnpzwaqwgjd5n9bwfajqft1hm U8awiv6rbrjmzvsum6mkym Usqp Cau
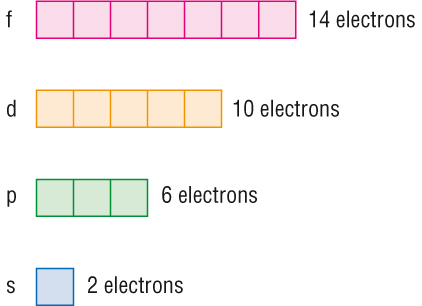
If Each Orbital Can Hold A Maximum Of 3 Electrons The Number Of Elements In The 4th Period Of The Periodic Table Long Form Is Socratic

Solved A Two Types Of Nodes Occur In Atomic Orbitals Sp Chegg Com

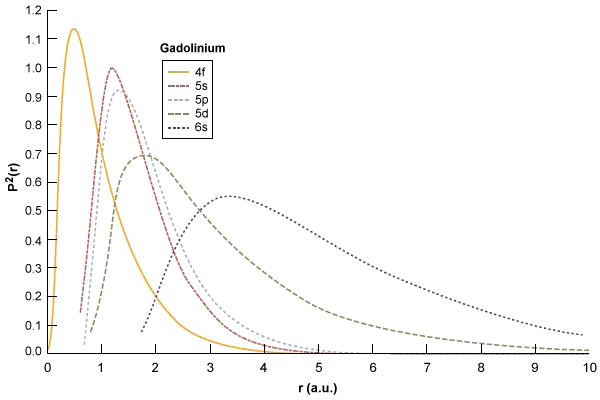
Square Of The Radial Wavefunctions For The 4f 5s 5p And 6s Energy Download Scientific Diagram

Effects Of Relativistic Motion Of Electrons On The Chemistry Of Gold And Platinum Sciencedirect

Within An Energy Level N 1 2 3 4 There Exists N Types Of Orbitals And N 2 Sublevels Norbital Types One S Orbital Three P Orbitals One S Orbital Ppt Download
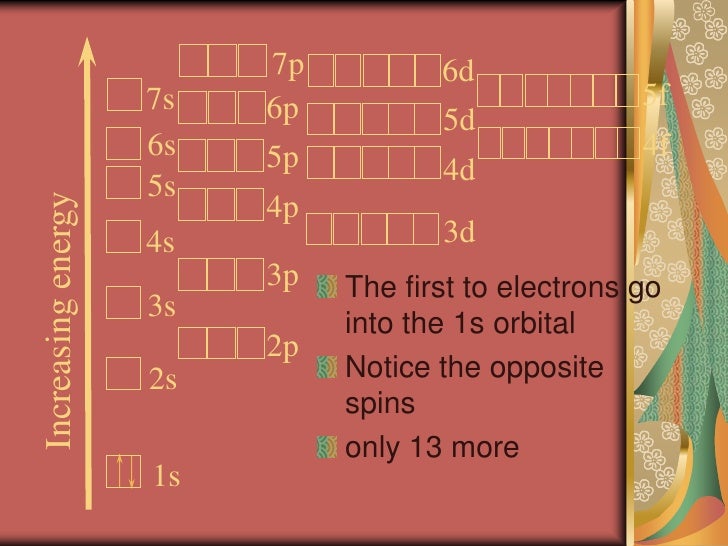
Bohr Model And Electron Configuration

Ap Chapter 6 Study Questions

What Is The Total Number Of Electrons Possible In The 6s Orbital

What Are The Maximum Number Of Electrons In Each Shell Chemistry Stack Exchange
Electrons Orbitals Ppt Download

Chemistry The Central Science Chapter 6 Section 9
Answer In Inorganic Chemistry Question For Mahesh Q A

Atom Orbits And Energy Levels Britannica
Q Tbn 3aand9gcq6txsjtuhsrh Clndtim11hdgl4ehavztrji6gbdqgur6segna Usqp Cau

Core Electron Wikipedia
:max_bytes(150000):strip_icc()/energylevels-56a129545f9b58b7d0bc9f39-5aeb7f1aae9ab800373981a3.png)
Electronic Structure And The Aufbau Principle

Di Tuujmcuous Use Of The Same When Electrons Are Added In The 6s Orbital What Happens To The Energy Level Of The 5d Orbitals As A Result After The Electron Enters 5d
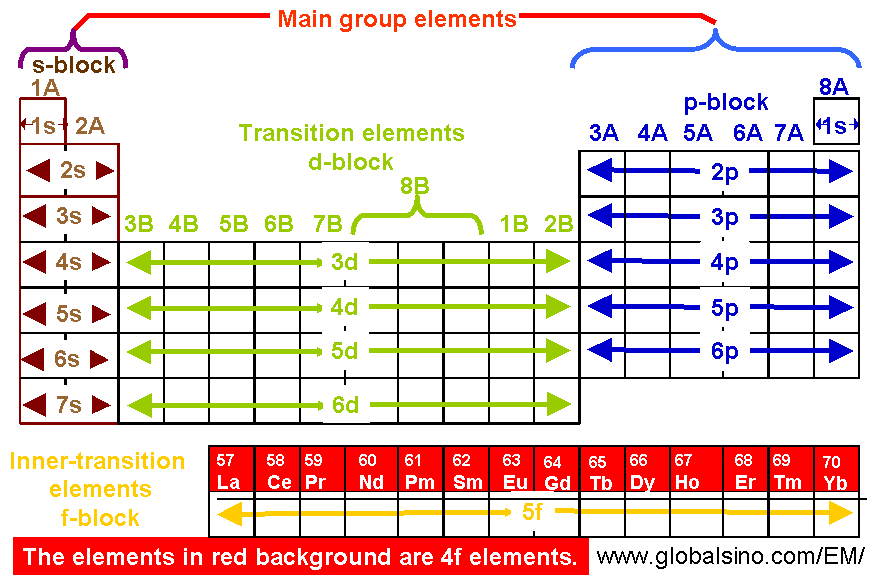
4f Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book

Electron Configurations
The Lanthanides

Quantum Numbers Video Quantum Physics Khan Academy
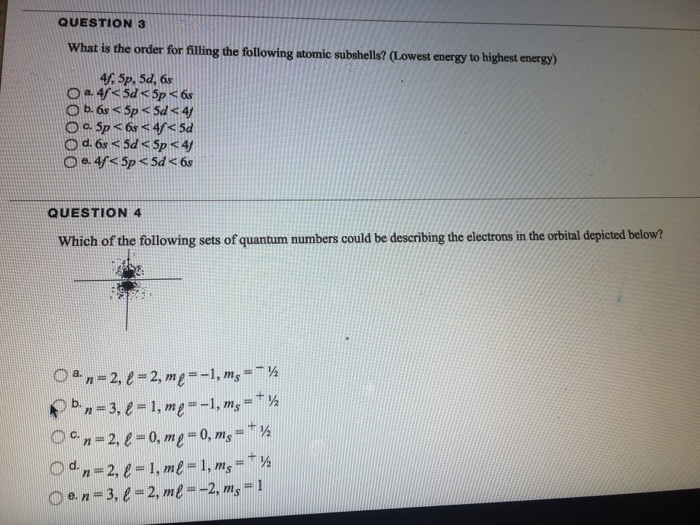
Solved What Is The Order For Filling The Following Atomic Chegg Com
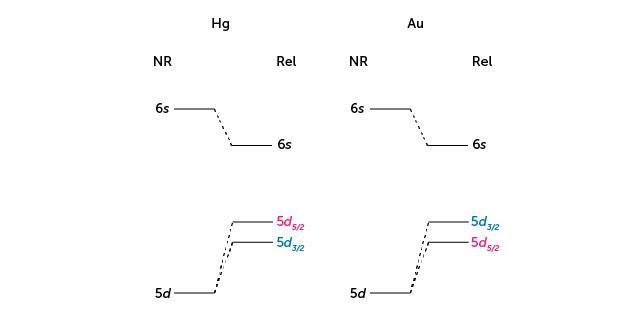
Is Relativity Creating Cracks In The Periodic Table Feature Rsc Education
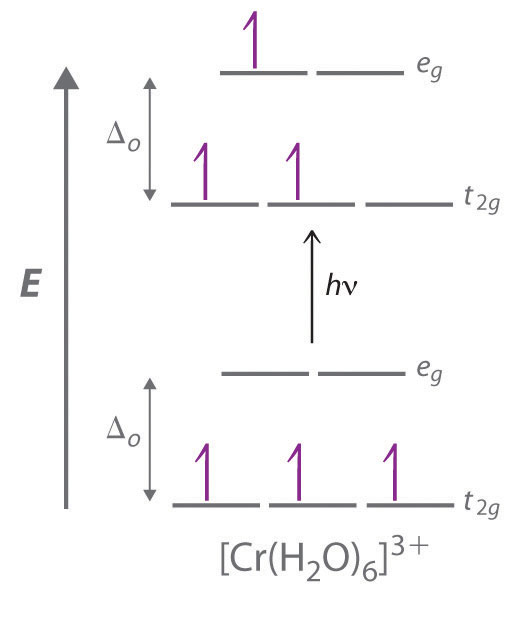
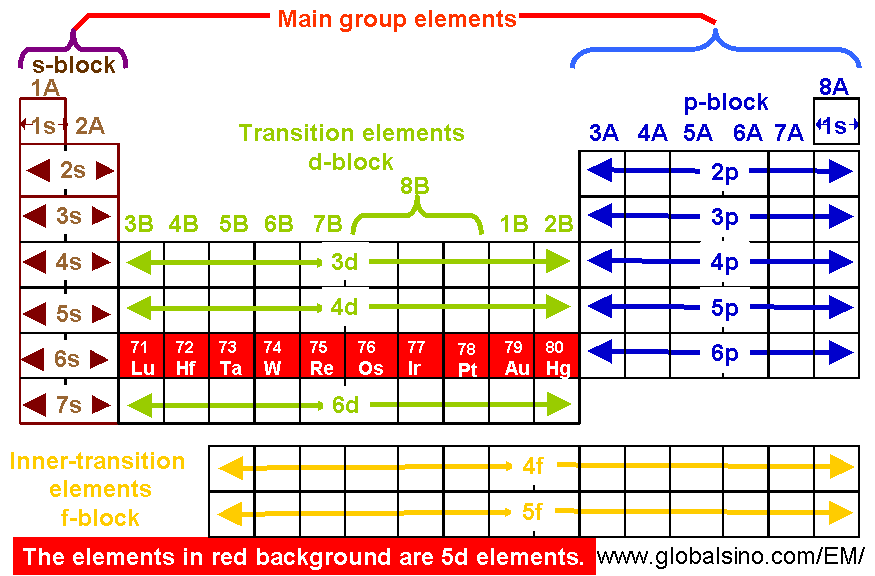
The D Block Elements
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry

Radial Distribution Of The 6s And 7s All Electron And Download Scientific Diagram
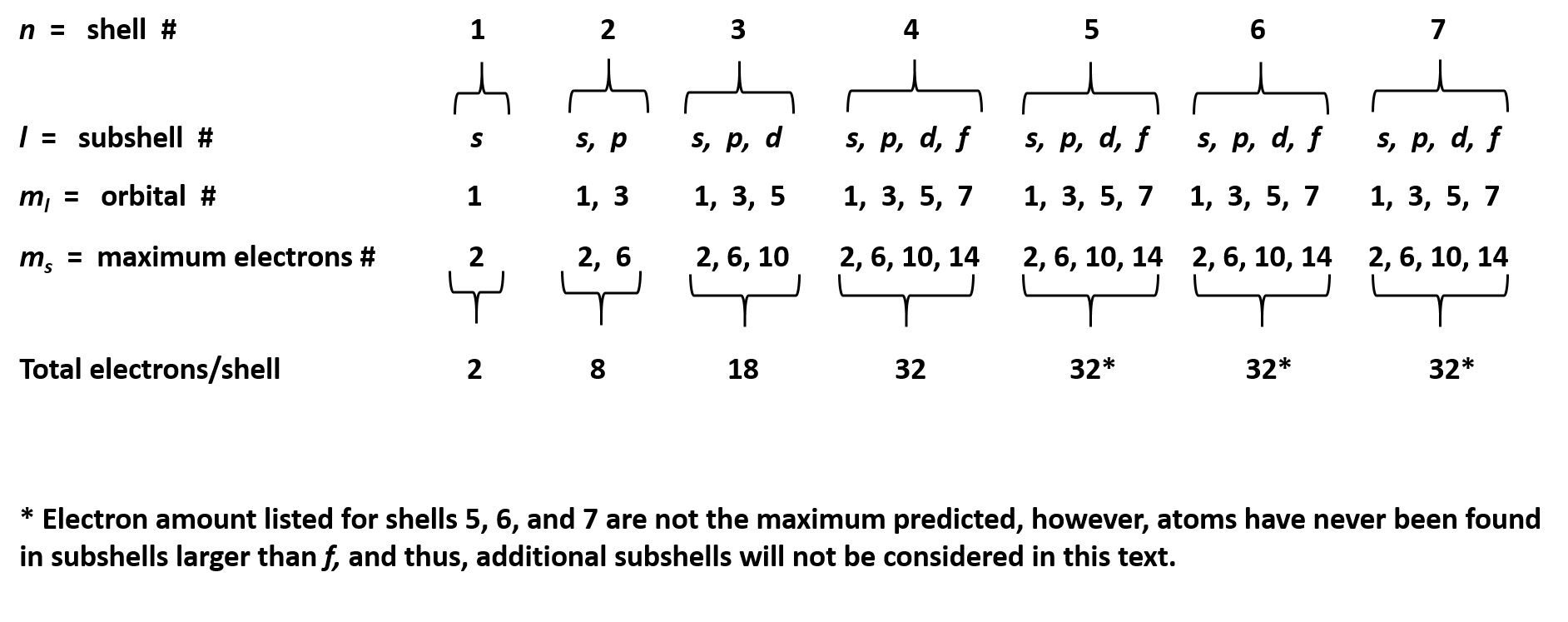
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

Chapter 9
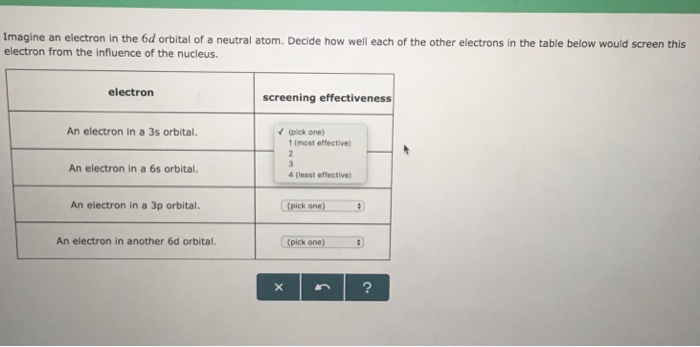
Solved Imagine An Electron In The 6d Orbital Of A Neutral Chegg Com
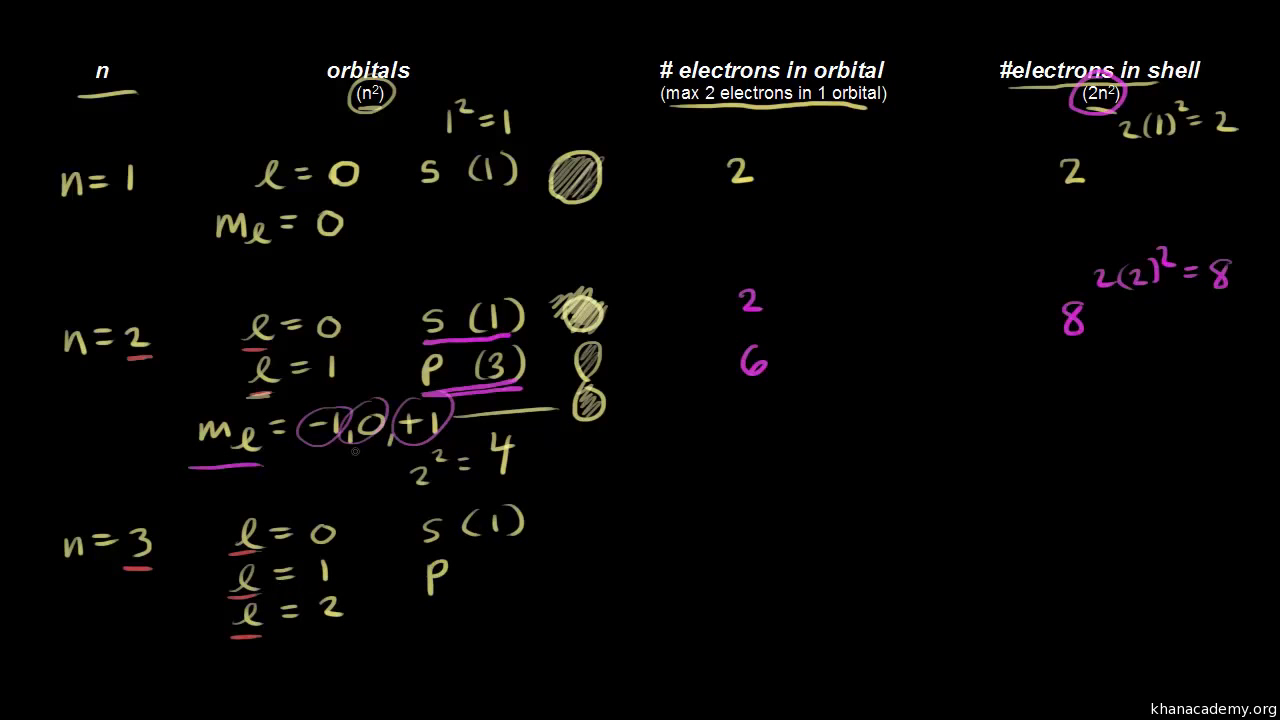
Quantum Numbers For The First Four Shells Video Khan Academy

Conventional Notation For Electronic Structure Electronic Structure Mcat Content

Quantum Numbers Atomic Orbitals And Electron Configurations
Strong Hybridization Between Bi 6s And O 2p Orbitals In Sillen Aurivillius Perovskite Bi4mo8x M Nb Ta X Cl Br Visible Light Photocatalysts Enabling Stable Water Oxidation Journal Of Materials Chemistry A
Q Tbn 3aand9gcrjaqjlb8lpy2zmnxb7djnungtx4z3mbsdjg7v7iofb5bmcmoq Usqp Cau

Imagine An Electron In The 6d Orbital Of A Neutral Atom Dec Clutch Prep

Shape Of The 6s Atomic Orbital On White Background Available Stock Photo Picture And Royalty Free Image Image 5717

8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts
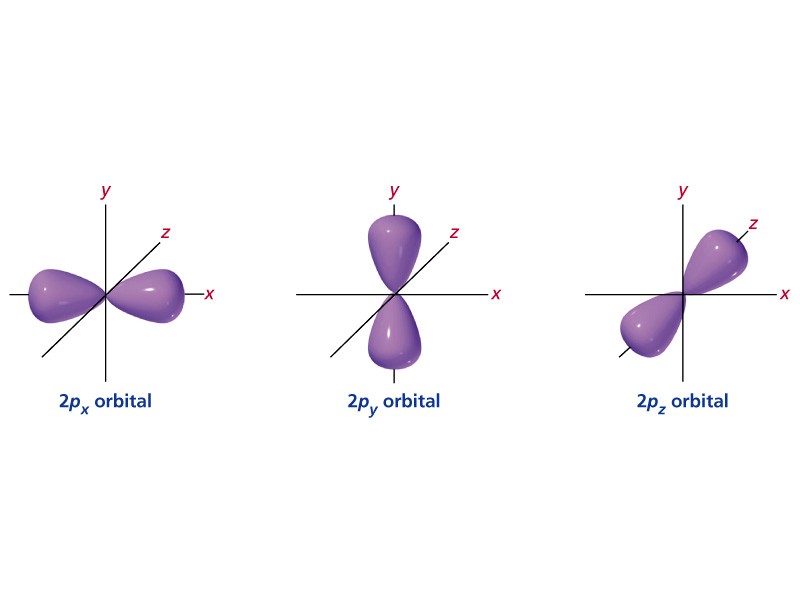
What Are The Four Quantum Numbers Example
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
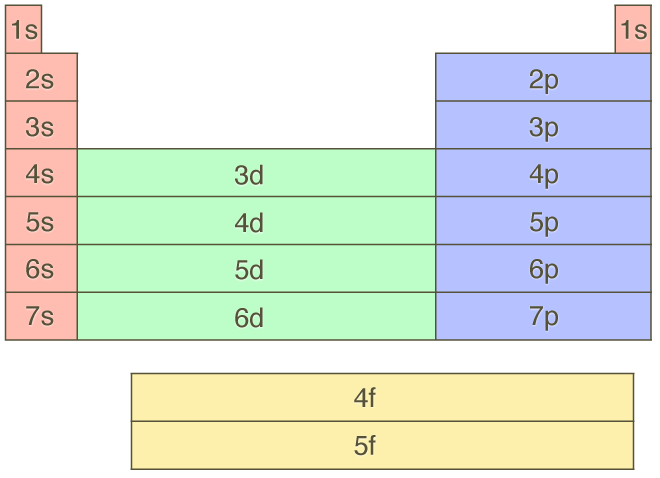
Aufbau Principle
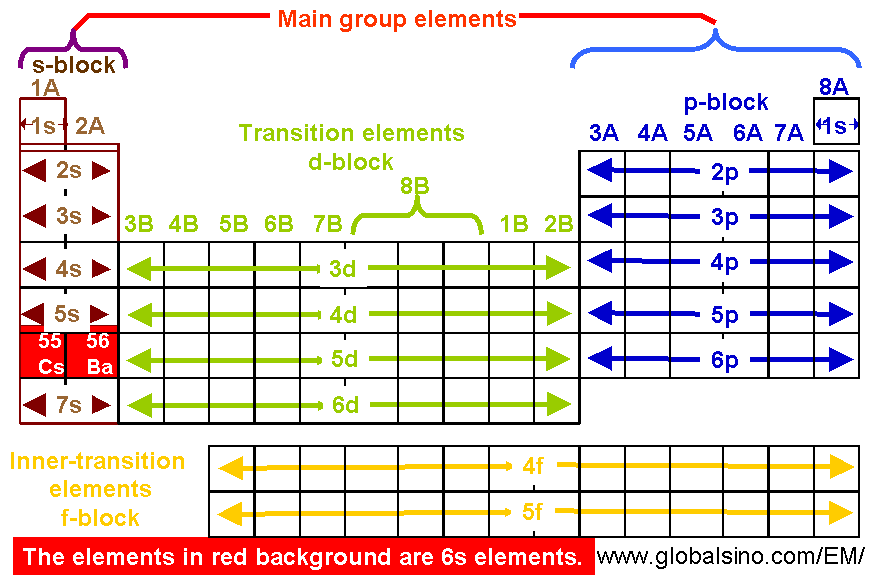
6s Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book

Principal Quantum Number An Overview Sciencedirect Topics
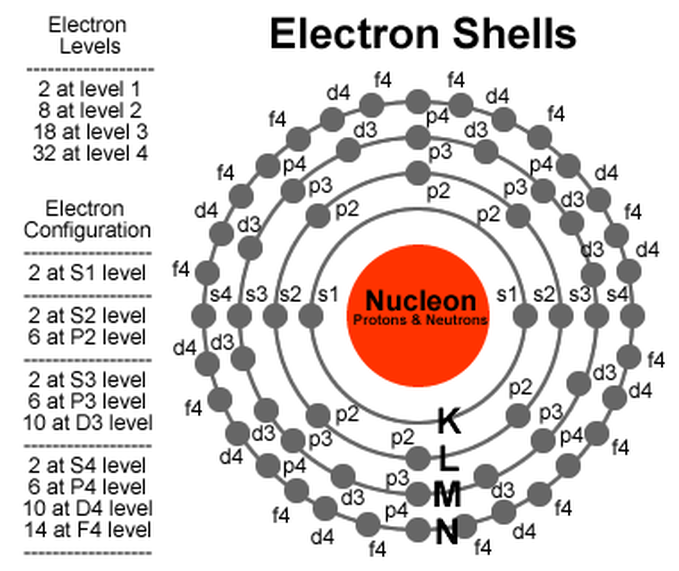
Electron Arrangement In Atoms Elements And The Periodic Table

Electron Configurations Of The 3d Transition Metals Video Khan Academy

It Fulus M Uus Used For The Same B When Electrons Are Added In The 6s Orbital What Happens To The Energy Level Of The 5d Orbitals As A Result After The
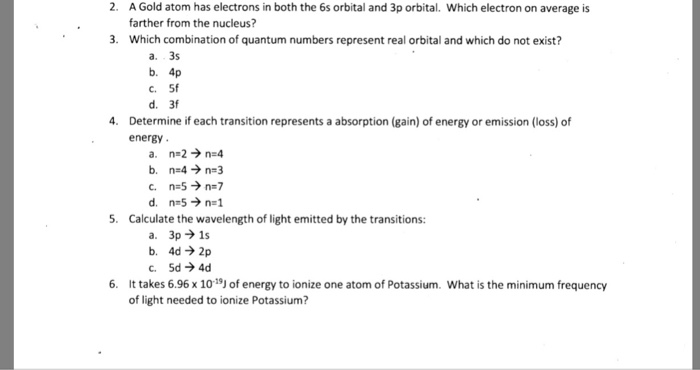
Solved A Gold Atom Has Electrons In Both The 6s Orbital A Chegg Com

Oneclass How Many Orbitals Does The 6s Subshell Have A 0 B 1 C 2 D 3 E It Varies What Is The Va

Quantum Number Wikipedia

Quantum Numbers Atomic Orbitals And Electron Configurations

Electron Configurations
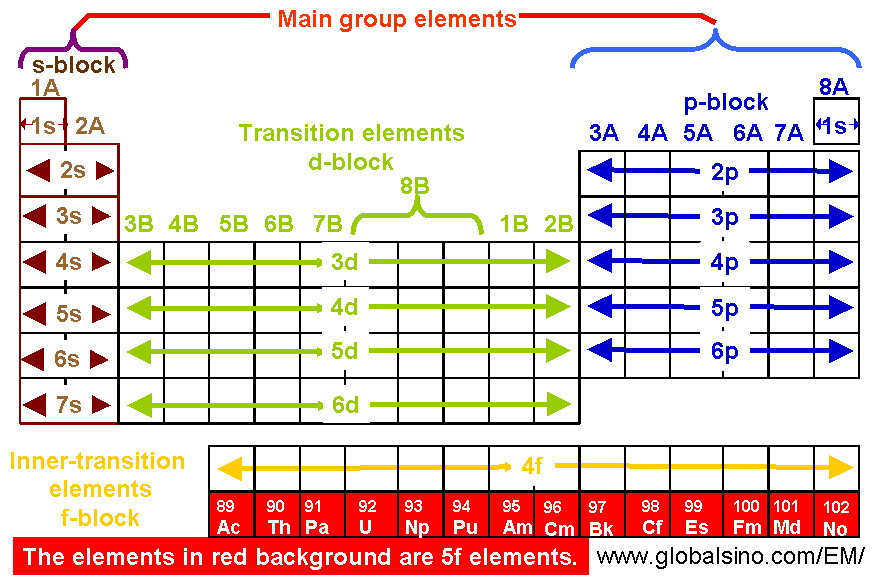
5f Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book

Unit 3 Study Guide Answers
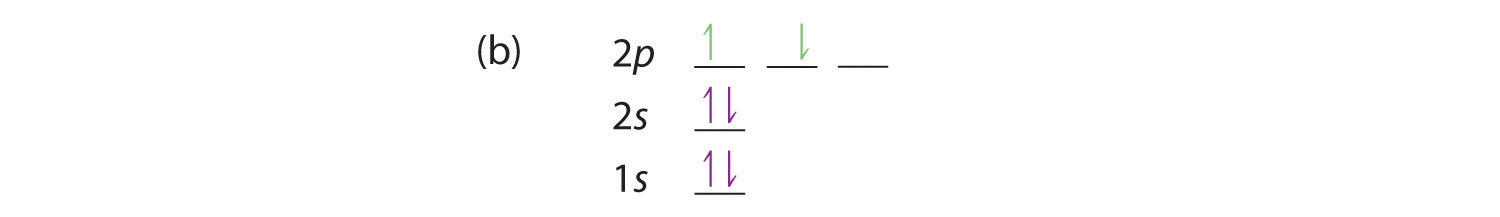
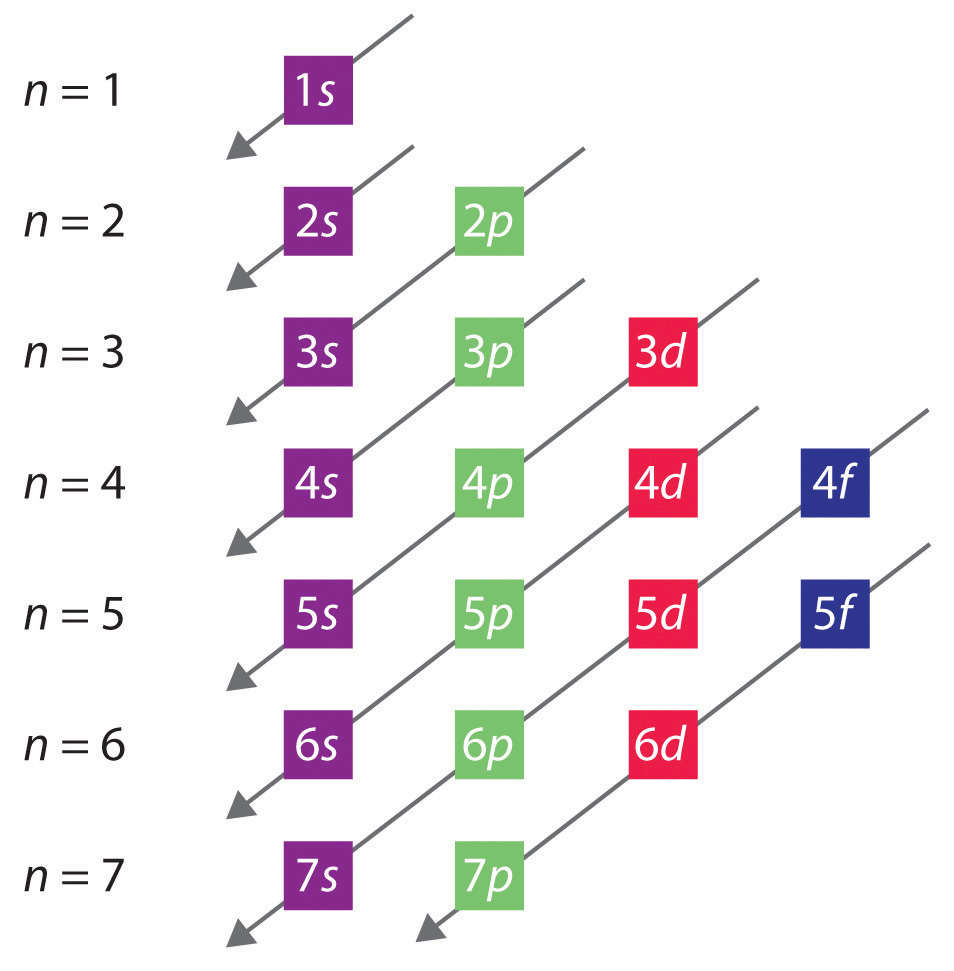
Building Up The Periodic Table
3 4 Electronic Structure Of Atoms Electron Configurations Chemistry Atoms First 2e Openstax

Electron Configuration Anomalies Villanova College Chemistry Blog

The Relativistic Contraction Of The 6s Orbital And Expansion Of The 5d Download Scientific Diagram

Aufbau Principle Wikipedia

Why Does Platinum S Electron Configuration Not Conform To Hund S Rule I E Have A Full 5d Orbital As Other Metals In Its Group Do E G Palladium Quora

Electron Configuration Wikipedia
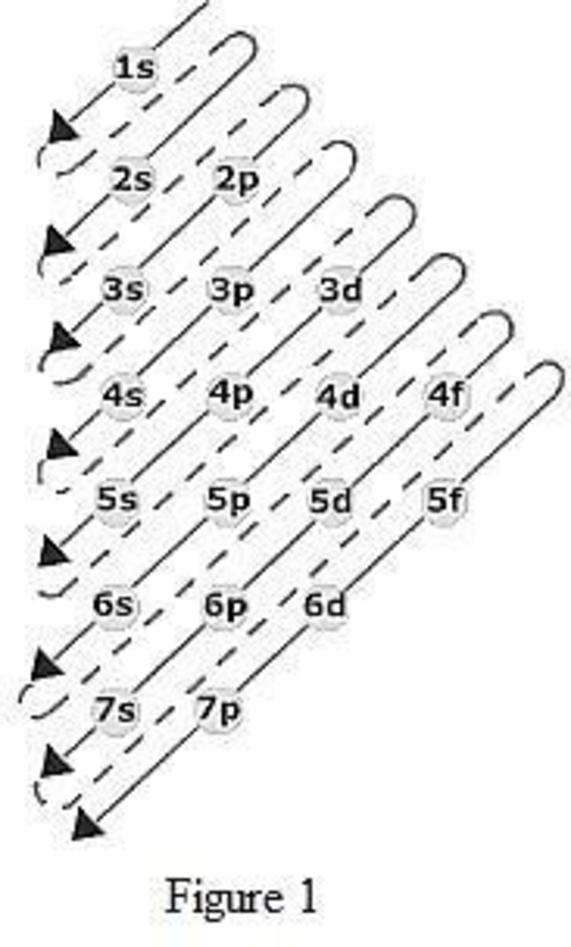
Building Up The Periodic Table
Q Tbn 3aand9gctoersax1tv2jzliwx6s5bncgkklg5nd3syvoykgyxyb68p8ybj Usqp Cau

Aufbau Principle Ck 12 Foundation
Draw The Orbital Diagram Associated With Each Of The Following Electron Configurations A 1 S 2 2 S 2 2 P 2 B 1 S 2 2 S 2 2 P 2
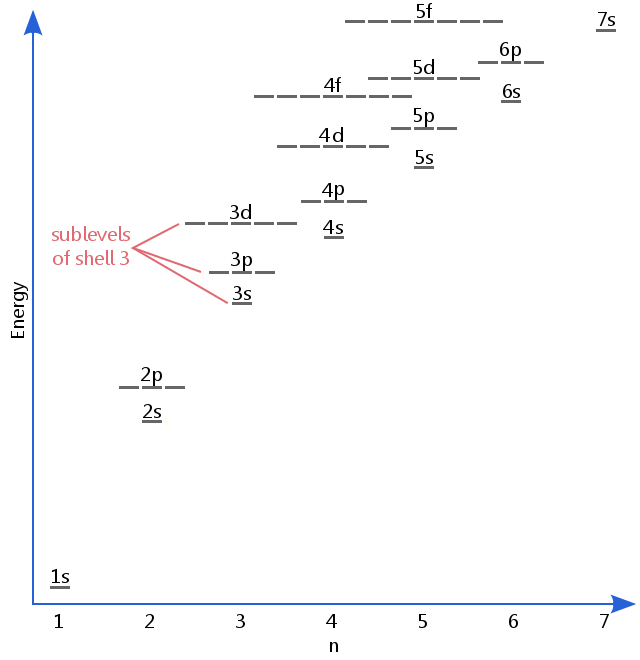
Definition Of Sublevel Chemistry Dictionary

Periodic Table The Basis Of The Periodic System Britannica
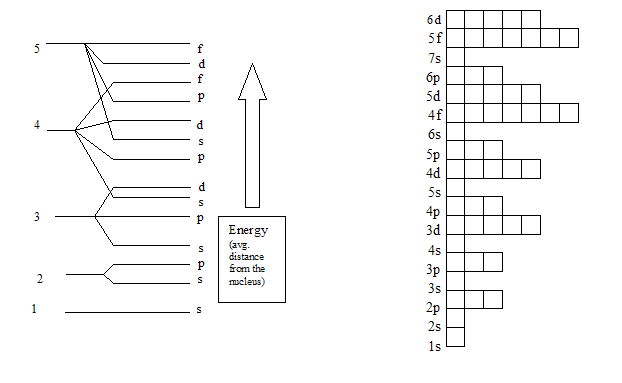
Summary Of Electron Configurations
Http Pnhs Psd2 Org Documents Lcasey Pdf
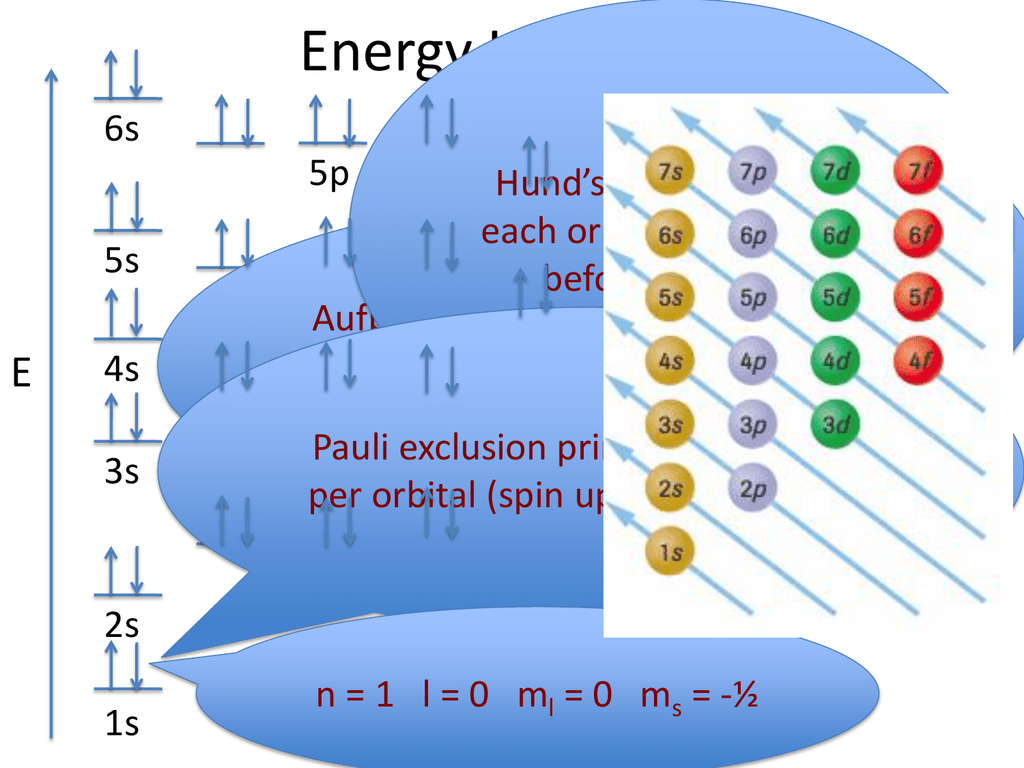
Electron Configuration

A Molecular Orbital Plots Of Ir 5d Orbitals In R 3 Irf 8 B Download Scientific Diagram
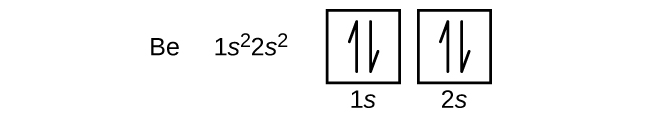
6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
Clarifying Electron Configurations Chemical Education Xchange



