Pcl3 Lewis Structure Resonance
Complete The Following Resonance Structures For Pocl 3 A Would You Predict The Same Molecular Structure From Each Resonance Structure B What Is The Hybridization Of P In Each Structure C
Covalent Bonding Electron Dot Diagrams Texas Gateway

Test Bank For Chemical Principles The Quest For Insight 7th Edition B
Solved Chemisary I Lab Nh3 Alence E Lewis Structure Form Chegg Com

Bonding Molecular Structure Ppt Download
Is Pcl3 Non Polar Or Polar Why Quora
1s^2 2s^2 2p^3 Now that we know the number of valence electrons per element, it is just a matter of drawing the electron dot configuration.
Pcl3 lewis structure resonance. Drawing the Lewis Structure for PCl 5. So the resonance structure on the left, and the resonance structure on the right, and some people disagreed with me, and said that's not the dot structure for sulfur dioxide. It is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds.
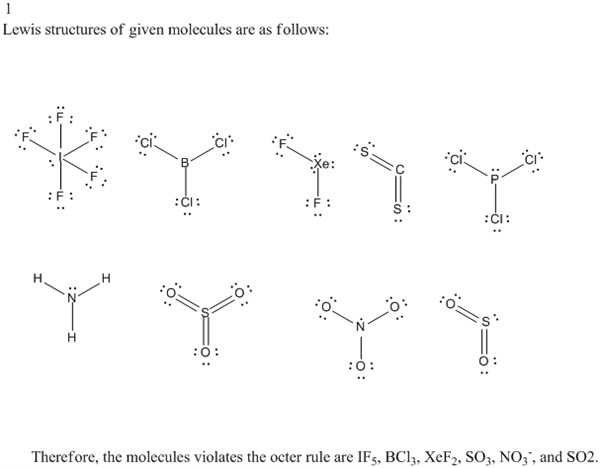
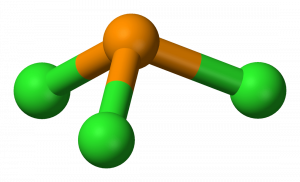
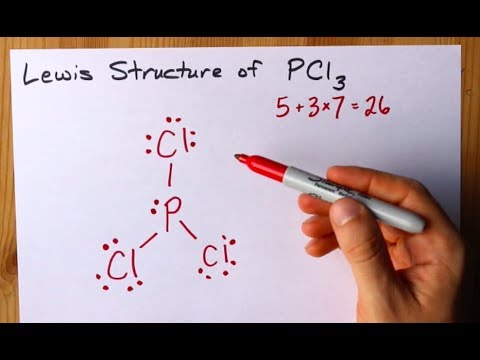
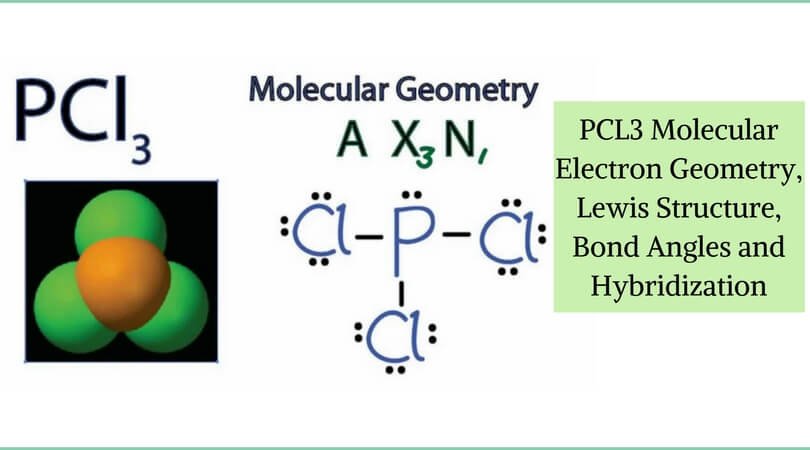
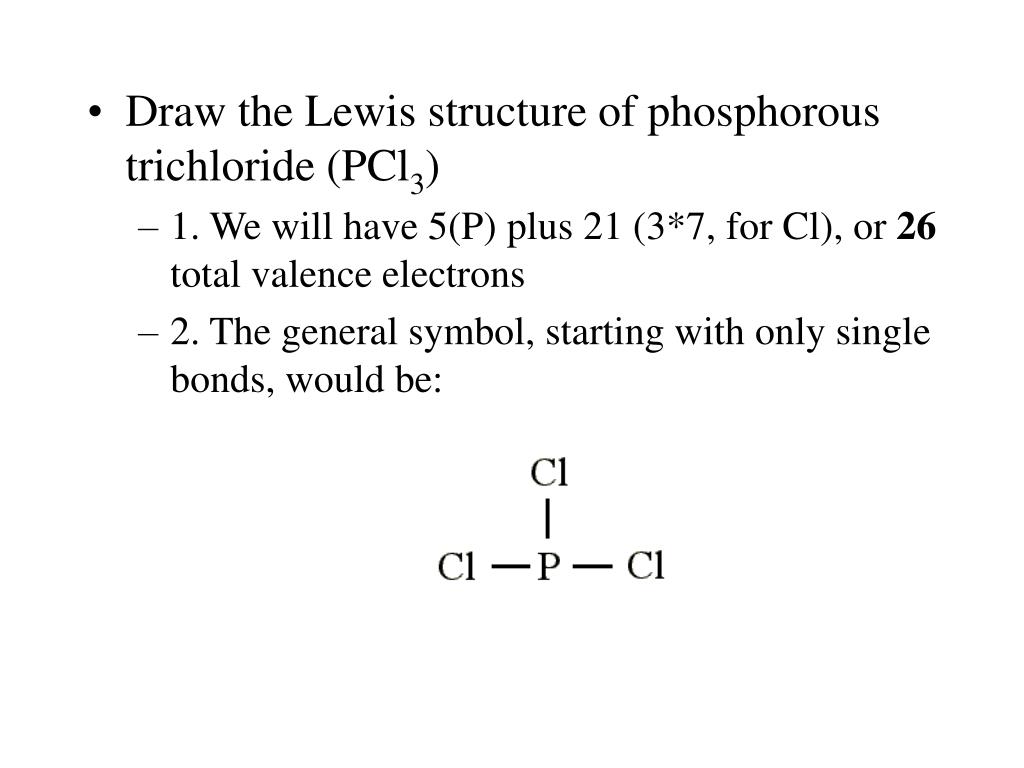
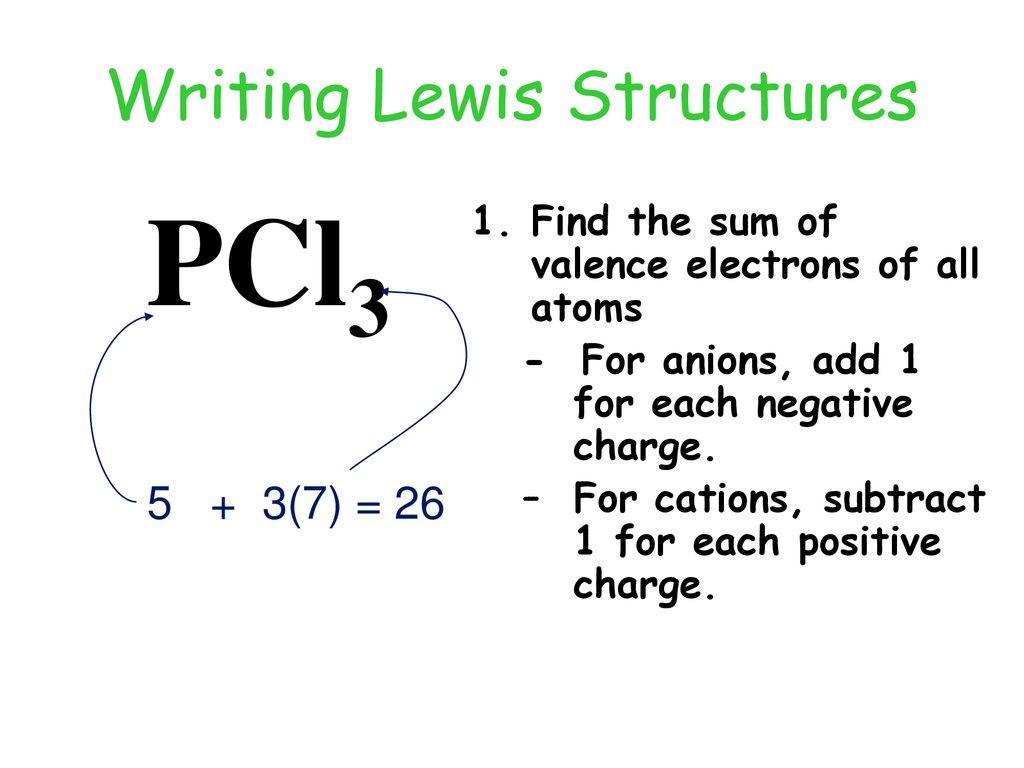
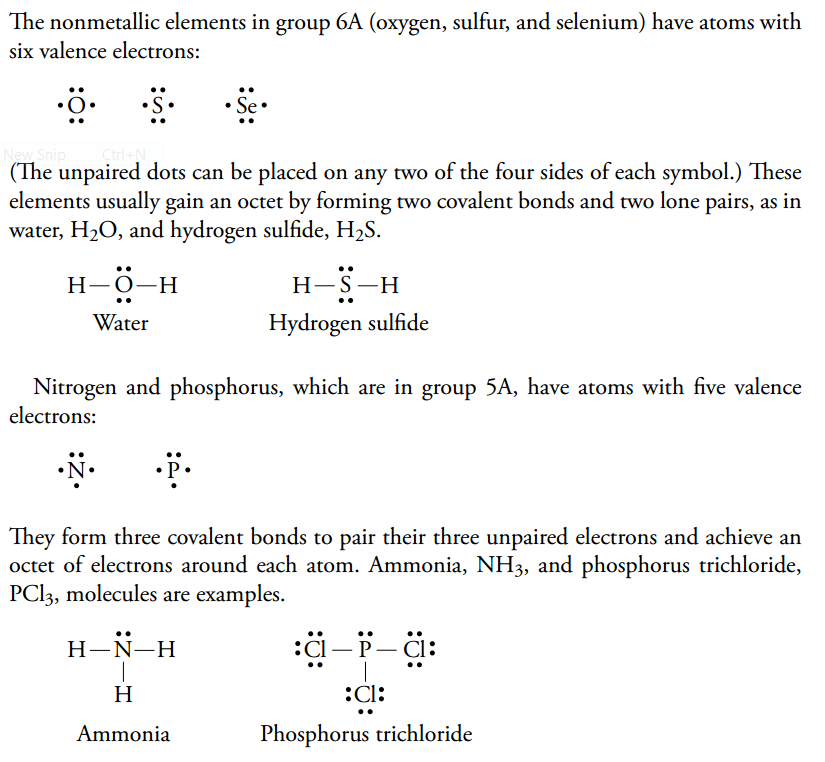
Exceptions to octet rule - odd # of electrons - not possible for octet to form around. For the PCl3 Lewis structure we first count the valence electron. It should be determined the following molecule which has trigonal pyramidal geometry.
These molecules have to one lone pair and three bond pair. However, when multiple equally valid structures can be drawn, these structures are called resonance structures. Determine which of these molecules has a central atom that unavoidably violates the octet rule.
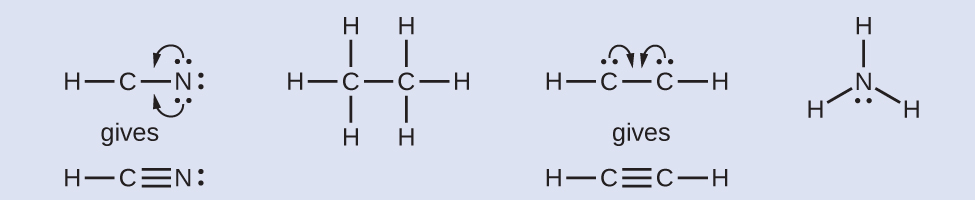
In tetrahedral geometry, the molecule like CH 4 has 4 bond pair s and no lone pairs. Resonance structures are a better depiction of a Lewis dot structure because they clearly show bonding in molecules. Resonance Compounds can have more than one Lewis structure that represents them correctly.
In the Lewis structure for elemental nitrogen, there is(are) A) a single bond between the nitrogens. C) a triple bond between the nitrogens. 3 resonance structures You must first know how many valence electrons are in one N atom.
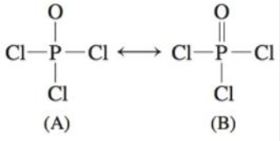
Once we know how many valence electrons there are in PCl5 we can distribute them around the central atom and attempt to fill the outer shells of each atom. They are not different compounds but they are drawn in different ways. The better ones have minimal formal charges, negative formal charges are the most electronegative atoms, and bond is maximized in the structure.
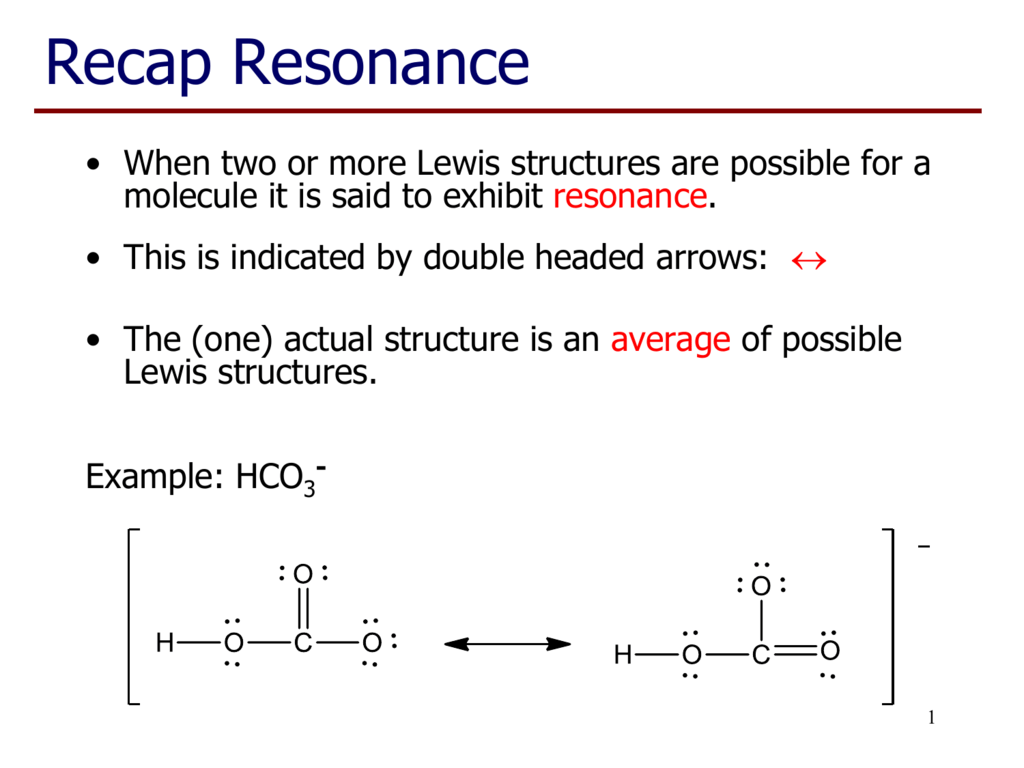
The actual distribution of electrons (the resonance hybrid) is an average of the distribution indicated by the individual Lewis structures (the resonance forms). The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorus.The heat of reaction, ca. The structures and bond.
Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written. In the PCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. A step-by-step explanation of how to draw the PCl3 Lewis Structure (Phosphorus Trichloride).
A) CO 2 b) CO. The position of the atoms is the same in the various resonance structures of a compound, but the position of the electrons is different. Double arrow used to show resonance structures ;.
Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written. In a Hoechst continuous process, molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. Indicate which has the strongest carbon-oxygen bond.
B) a double bond between the nitrogens. Described by two resonance structures that average the two C–O bonds. Bent shape, here 2 lone pair and 2 bond pairs.
A valid lewis structure of _____ cannot be drawn without violating the octet rule. By writing the electron configuration, we know that the number of electrons in its outermost shell (2s 2p) is 5. 10 times the heat of evaporation, keeps the system at its boiling point, and the phosphorus trichloride distills off.
Which statement best describes the bonding character of this ion?(a) One double b. Resonance structures differ by. C2H2 C2H4 C2H6 b) Based on your Lewis structures for the postlab assignment, which molecules below have a three dimensional structure?.
(i)PCl3 (ii)CH2Cl2 (iii)HCN (iv)C2H4 (v)NH3 1)In the Lewis structures of _____ the central atom has one lone pair of electrons. In the solid state at -164°C, P-F (axial) is 158.0 pm and P-F (equatorial) is 152.2 pm. Actual structure an average of the possible Lewis resonance structures ;.
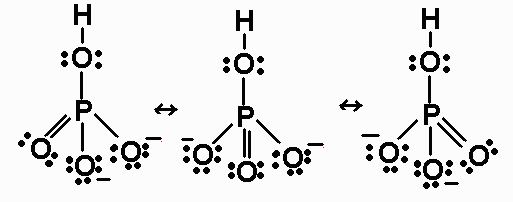
Draw the Lewis structures for BrO3- and indicate the formal charges for Br and O’s.a) Indicate the total number of valence electronsb) Describe the preferred Lewis structure.c) How many resonance structures are there in the ion?d) Give the name of the ion. In the previous section, we discussed how to write Lewis structures for molecules and polyatomic ions. The actual distribution of electrons (the resonance hybrid) is an average of the distribution indicated by the individual Lewis structures (the resonance forms).
A formate ion does not possess resonance structures, as there is only one Lewis structure to describe it. As we have seen, however, in some cases, there is seemingly more than one valid structure for a molecule. E.g NH 3 has trigonal pyramidal geometry.
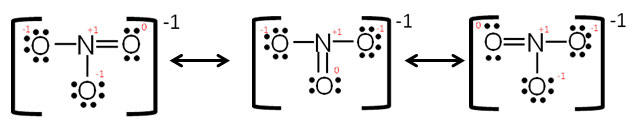
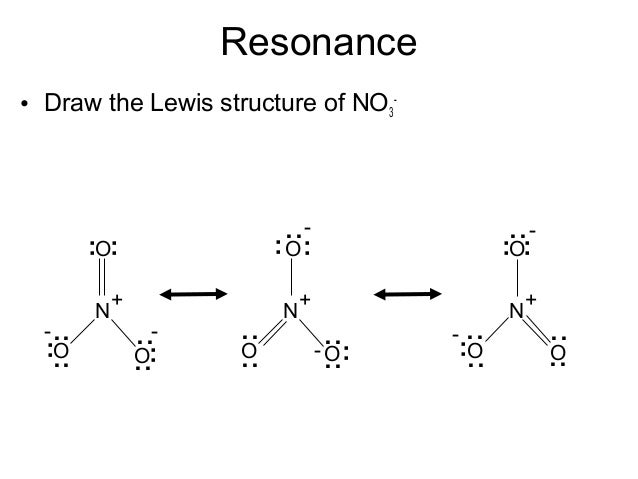
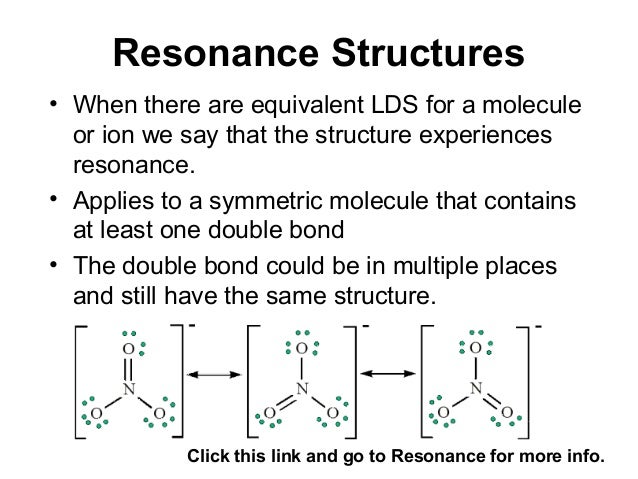
The individual lewis structures are called resonance forms. In each of the three structures in the middle, "S" has a formal charge of +1 and one of the "O" atoms has a formal charge of -1. Draw the Lewis structures for three resonance forms of nitrate, NO 3-.
It is the well-known fact that if there is a vast difference of the electronegativity, there are more chances of polarity. Resonance occurs in cases where two or more Lewis structures with identical arrangements of atoms but different distributions of electrons can be written. The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O.
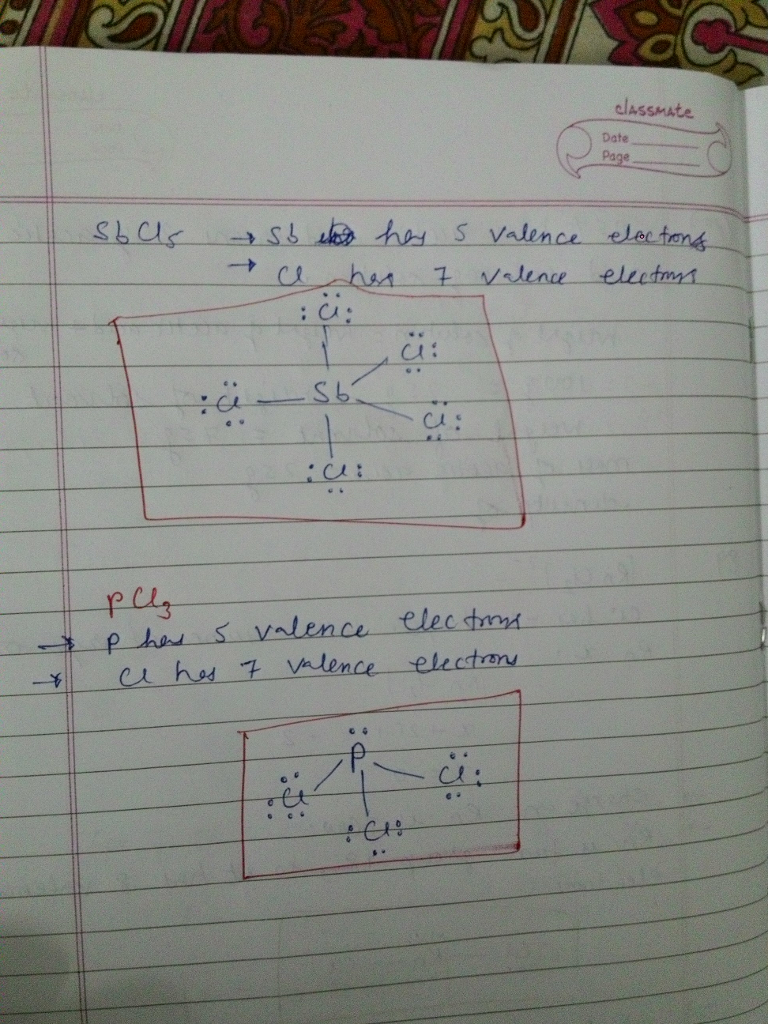
It has uses in determining possible electron re-configuration when referring to reaction. Five pairs will be used in the chemical bonds between the P and Cl. Write the Lewis structures for the following, and include resonance structures where appropriate.
The Lewis structure shown below suggests that O 2 is diamagnetic because all electrons are paired:. How many electrons are in the Lewis structure for SO 2?. The Lewis structure of N2H2 shows _____.
The actual distribution of electrons (the resonance hybrid) is an average of the distribution indicated by the individual Lewis structures (the resonance forms). There is more than one answer and you must choose all of the molecules in the list that exhibit resonance.) Options:. Pure Chlorine Nuclear Quadrupole Resonance and Low Temperature F 19 Nuclear Magnetic Resonance Spectra.
PCl3 C) SO3 D) CCl4 E. It has a trigonal pyramidal shape, owing to the lone pairs on the phosphorus. The Lewis structure uses dots to represent the valence electrons.
In this case, each chlorine has seven valence electrons. Molecular Structures of PCl 4 F, PCl 3 F 2, and PCl 2 F 3:. Hree pairs will be used in the chemical bonds between the P and Cl.
A) HCl b) CF 4 c) PCl 3 d) PF 5. There is more than one answer and you must choose all of the molecules in the list that exhibit resonance.) Options:. In short because the electron distribution in NO2 is an average of those 3 resonance forms, the distribution of electrons in the bond of N and O may be seen to be greater than that of.
C) the observed structure is an average of the resonance forms D) the same atoms need not be bonded to each other in all resonance forms E) there cannot be more than two resonance structures for a given species For the questions that follow, consider the BEST Lewis structures of the following oxyanions:. Indicate which of the three has the strongest carbon-oxygen bond. Drawing the Lewis Structure for PCl 5.
A single Lewis Structure of the formate ion (HCO 2-) is shown below. Explain the concept of resonance and draw Lewis structures representing resonance forms for a given molecule \n. Cs2, PCl3, SO2, SO3, O3, NH3, BCl3, and IF5.
🤓 Based on our data, we think this question is relevant for Professor Smith's class at UH. A) ozone (left) and b) carbonate (right) The total bond energy of a substance for which resonance structures are written is greater than would be. Molecule PCl 3 CO 2 H 2 S Lewis structure (b) The following table shows the Lewis structures and bond angles for the molecules SO2 and H 2 CO.
The total number of electrons involved is calculated. In each of them, "S" has a formal charge of +2 and two of the "O" atoms have formal charges of -1. In terms of Lewis structures, formal charge is used in the description, comparison, and assessment of likely topological and resonance structures by determining the apparent electronic charge of each atom within, based upon its electron dot structure, assuming exclusive covalency or non-polar bonding.
There are seven resonance structures for "SO"_3. For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table. A valid lewis structure of _____ cannot be drawn without violating the octet rule.
However, when chemists studied the 19 F NMR spectrum of PF 5 , they saw only one signal, even at -100°C, showing there was only one environment for fluorines. For the PCl3 Lewis structure we first count the valence electron. In the Lewis structure for PCl 3 there are a total of 26 valence electrons.
Cs2, PCl3, SO2, SO3, O3, NH3, BCl3, and IF5 2. When resonance is possible, only one of the possible resonance structures is necessary to predict the correct structure because all resonance structures give the same structure. Equivalent Lewis dot structures, such as those of ozone, are called resonance structures A Lewis electron structure that has different arrangements of electrons around atoms whose positions do not change.
- Voiceover In the previous video, we looked at the dot structure for sulfur dioxide, and I drew out two resonance structures. The phosphorus has an electronegativity value of 2.19 and chlorine comes with 3.16. Resonance structures have the same number of electrons and therefore have the same overall charge.
The biggest difference between structures are where the electrons are placed in each one. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. PCl 5 is similar to PBr 5 and PF 5.If you can do those Lewis structures PCl 5 will be easy.;.
Find more Chemistry widgets in Wolfram|Alpha. A) 16 B) 30 C) 18 D) E) 32 19. A)(i) only B)(i) and (iv) C)(ii) and (iv) D)(i) and (iii) E)(i) and (v) 2)In the resonance form of ozone shown below, the formal charge on the central oxygen atom is _____.
By sharing an electron from each chlorine, both atoms can accumulate the eight electrons they need to have a full octet. This system facilitates visualization of the number of electrons an atom needs to satisfy its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons.
Resonance structures - placement of electrons changes, but atom placement doesn’t. Determine the formal charge of each element in the following:. Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having the chemical formula PCl 3.It is a toxic and volatile liquid which reacts violently with water to release HCl gas.
A)+2 B)-1 C)-2 D)+1 E)0. (In other words, which of the following molecules exhibit resonance?. The molecular geometry of PCL3 is trigonal pyramidal with the partial charge distribution on the phosphorus.
D) three unpaired electrons. A) Based on your observations during lab, which molecules below have a three dimensional structure?. Gas phase PF 5 molecules have a D 3h structure (P-F (axial) 158 pm and P-F (equatorial) 153 pm ;.
A) a nitrogen-nitrogen triple bond B) a nitrogen-nitrogen single bond. The Lewis structures are in Exercises 87 and 93. E) none of these 18.
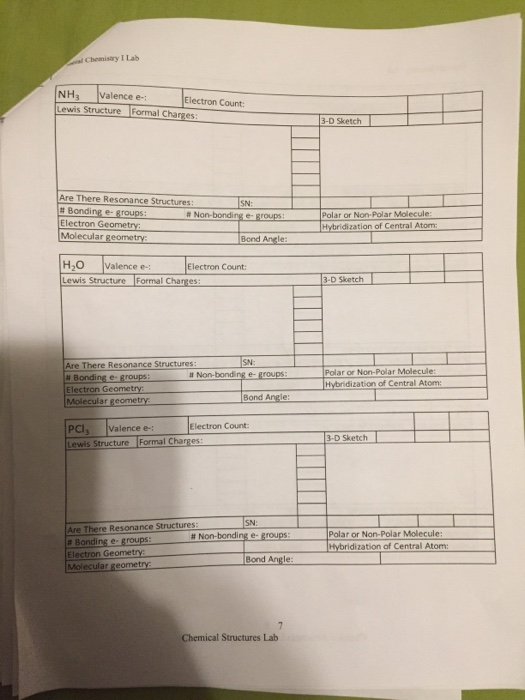
These are called resonance structures or resonance forms. H2S CO2 PCl3 NO21- SO3 CH2Cl2 c)Based upon your Lewis structures for the postlab assignment, which molecules below have multiple Lewis resonance structures that. Write the Lewis structures for the following, and include resonance structures where appropriate.
And the actual electronic structure of the molecule is called resonance hybrid of the resonance forms. Lewis dot diagrams are often employed to visualize the covalent bonding between atoms in a compound. We can use the concept.
Similarly, the three resonance structures of carbonate in Fig.2(b) are needed to account for the experimental fact that all three oxygens are equidistant from the central carbon. Cannot be described by a single Lewis structure ;. In the PCl 5 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center.;.
All the resonance structures are observed in various proportions C) the observed structure is an average of the resonance forms D) the same atoms need not be bonded to each other in all resonance forms. Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Gdem6cbab8 o47jqg3390ijjq bj26f5jjg99b v6fvr1m33r 4skbmlkoondzbhz 8zyobumo1c l840zgiqu9t 49rmxqar24 52jc6aonz413 cglhzmrhbj91s cpe3gg0zdknniw e5fgat40mkl 8i7svdndy0 rocsamu5vflr b75zxehtovz f6408qq6ezwa r8c355zmtjy8q 1fz2fwilb8jxl18 0r71ooyegjh4m b92wj7t2epihq6 8t9bev9318 g0gplalguymn008 vxqnimfam7i.
Determine which of these molecules has a central atom that unavoidably violates the octet rule. View Answer The ion, PO4^(3-), possesses more than one resonance structure. However, we know from experiment that O 2 is, in fact, paramagnetic due to unpaired electrons.
The first step always is to draw a valid Lewis structure when predicting molecular structure. In the Lewis structure for PCl 5 there are a total of 40 valence electrons. (In other words, which of the following molecules exhibit resonance?.
Carter Jr., and ;. Not all resonance structures are equal there are some that are better than others.

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube

Bonding Pp Flashcards Quizlet
Solved 1 Look Again At The Lewis Structures That You Dre Chegg Com

Reaction Of Alcohols With Pcl5 And Pcl3 Chemistry Stack Exchange

Lewis Structures

Which Of These Molecules Are Polar Check All That Apply H So2 Co2 Ch2ci2 H Pcl Homeworklib

Problem Set 6
Provide The Lewis Dot Structure And All Resonance For The Ion Rncl2 2 Sbcl5 Pcl3

Draw A Second Resonance Structure For The Following Radical Shown Below Study Com

Pcl5 Lewis Structure
7 3 Lewis Symbols And Structures Chemistry

Which One Of The Following Molecules Has Two Lone Pairs Of E Clutch Prep

Provide The Lewis Dot Structure And All Resonance For The Ion Rncl2 2 Sbcl5 Pcl3
Http Mrlawsonscience Weebly Com Uploads 1 0 9 5 Worksheet Lewis Structures Final W Ans Pdf
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Ch 9 Molecular Shape Bonding Patterns Polarity Dipole Ppt Download

Chapter 10 Flashcards Quizlet
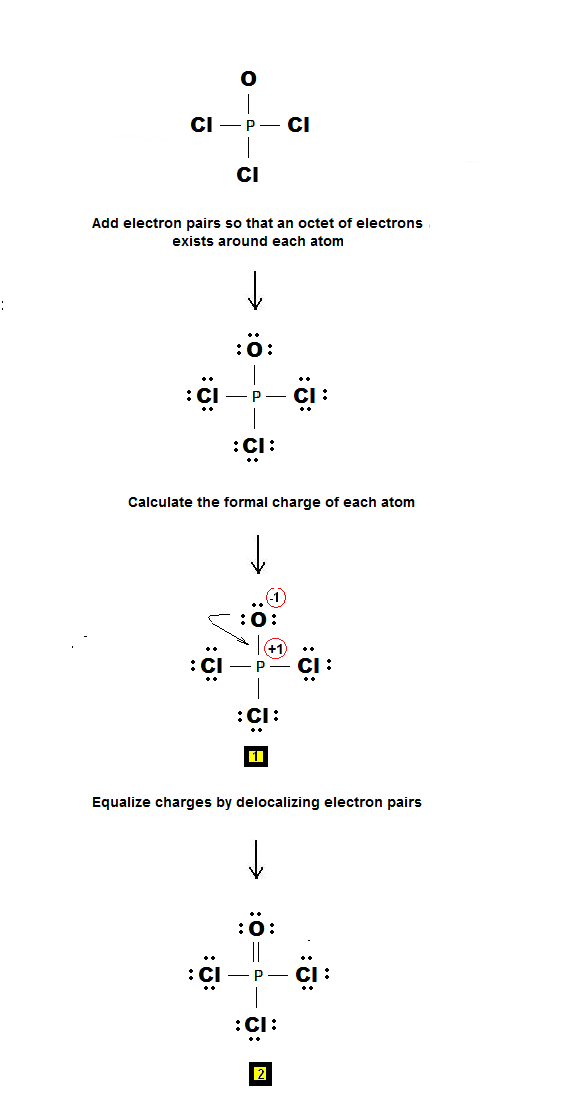
Chemistry Net How To Draw Lewis Dot Structures Pocl3 Phosphorous Oxychloride

Pcl3 Lewis Structure How To Draw The Lewis Structure For Pcl3 Phosphorus Trichloride Youtube

What Is The Lewis Dot Structure For Pcl3 Study Com

Chemistry 114 Chapter 10 Flashcards Quizlet

Pcl3 Lewis Structure How To Draw The Lewis Structure For Pcl3 Phosphorus Trichloride Youtube

Drawing Lewis Dot Diagrams Resonance Ppt Download

Explain The Structure Of Pcl3 And Pcl5 Chemistry Topperlearning Com C9yvmoxx
Phosphorus Trichloride Pcl3 Pubchem
Chap1

Problem Set 6

Chapter 8 Lecture Powerpoint
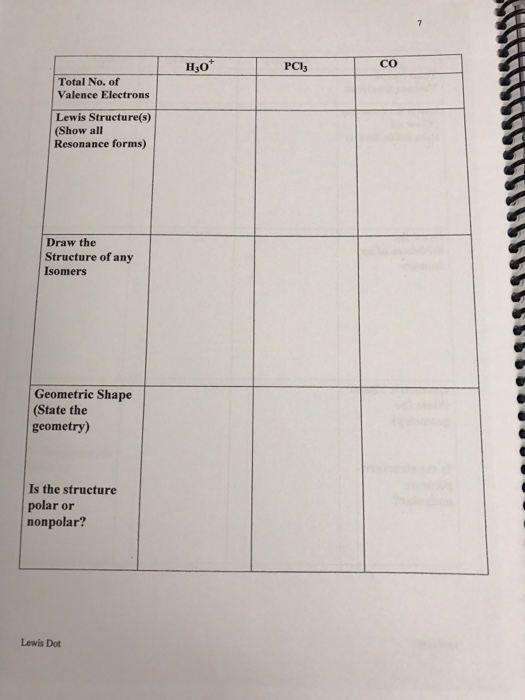
Solved H30 Pcl3 Total No Of Valence Electrons Lewis Stru Chegg Com
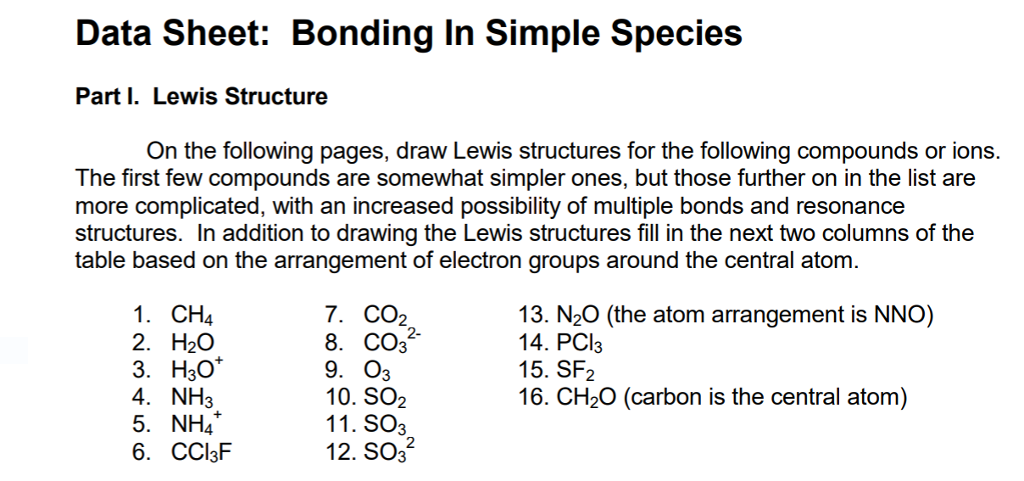
Solved Data Sheet Bonding In Simple Species Part I Lewi Chegg Com

Covalent Bonding Lewis Structures Ppt Video Online Download
Http Sites Uci Edu Chemcommonfinal Files 16 03 Midtermii Example Pdf
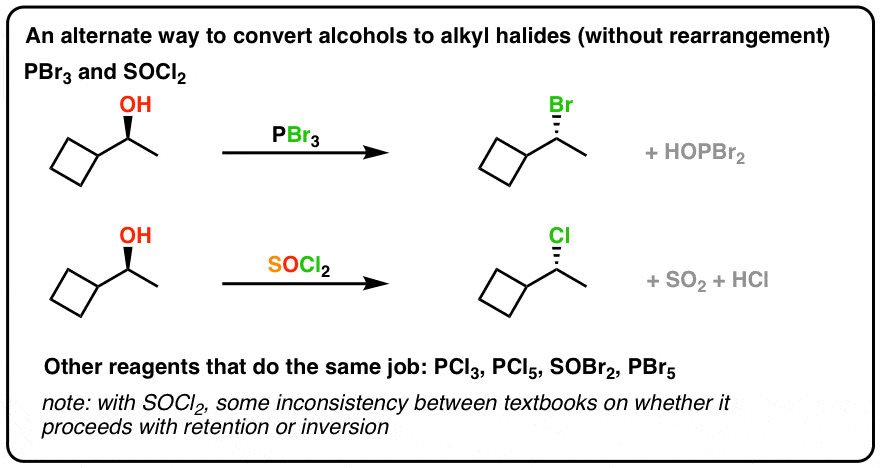
Pbr3 And Socl2 Master Organic Chemistry

Pptx

Chapter 10 Flashcards Quizlet
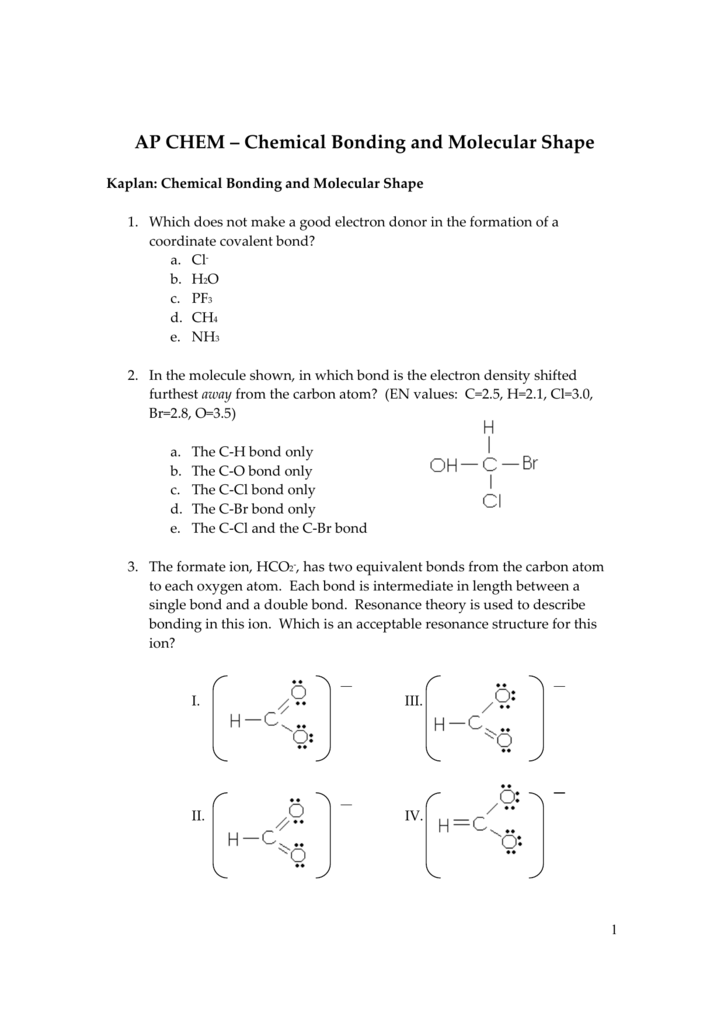
09 Ap Chemical Bonding
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure And Molecular Geometry Youtube
Untitled

How To Draw The Lewis Structure Of Pcl5 Phosphorus Pentachloride Youtube

Ccl2o Lewis Structure How To Draw The Lewis Structure For Ccl2o Youtube
Ppt Chemical Bonding I The Covalent Bond Powerpoint Presentation Free Download Id

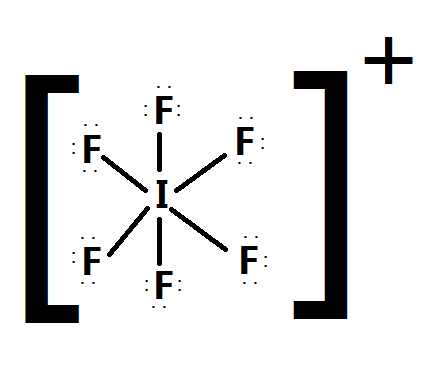
I3 Lewis Structure Shape Hybridization And Polarity
Akdjhfadklhjkhdfhj
Piazza Com Class Profile Get Resource Iog601eydm5tk Is0shpzcbbg636
Q Tbn 3aand9gcqd4mttlm Lxof4eicktwu61s1r1uqgmy1pgwcrao8cnbdkit O Usqp Cau

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Resonance Structures For No3 Nitrate Ion Youtube

Vsepr Theory Molecule Shapes Video Lesson Transcript Study Com

Phosphorus Pentachloride Wikipedia

Chemistry 1e03 Tutorials

Why Is Bf3 Written In Two Ways Quora
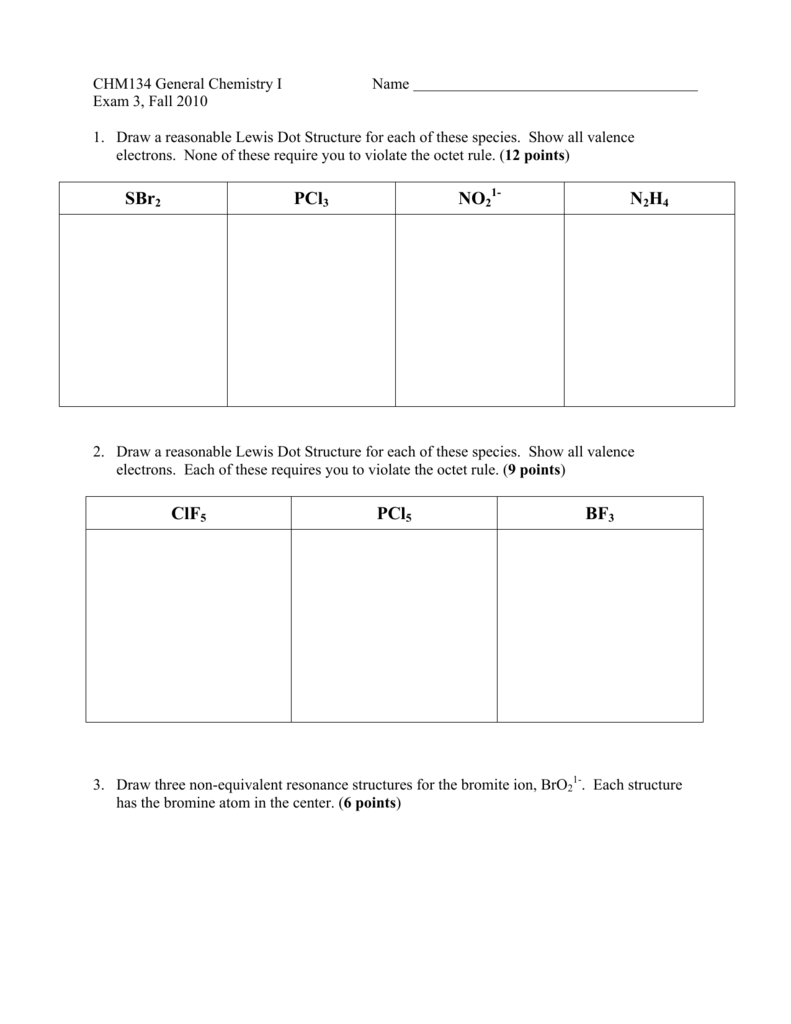
Sbr2 Pcl3 No2 N2h4 Clf5 Pcl5 Bf3

Two Major Contributing Resonance Structures Are Possible For The Following Anion One Is Given Below But Is Incomplete Complete The Given Structure By Aiding Non Bonding Electrons And Formal Charges Study Com
Http Sites Uci Edu Chemcommonfinal Files 16 03 Midtermii Example Pdf
Q Tbn 3aand9gcsrp00oizxf0ejlxa4ycljjn4 Ch4cu1vuw3gfnrzg Usqp Cau

Solved Question 1 Select The Molecule Below That Has Reso Chegg Com
Polarity Lewis Structures And Resonance Ppt Download
Q Tbn 3aand9gcseyobbyyxbjn6tvzukgg8pqxexr4l9yuq G9cwemw2k9xszngu Usqp Cau

Exceptions To The Octet Rule Chemistry Master

Phosphorous Trichloride Pcl3 Lewis Dot Structure Youtube
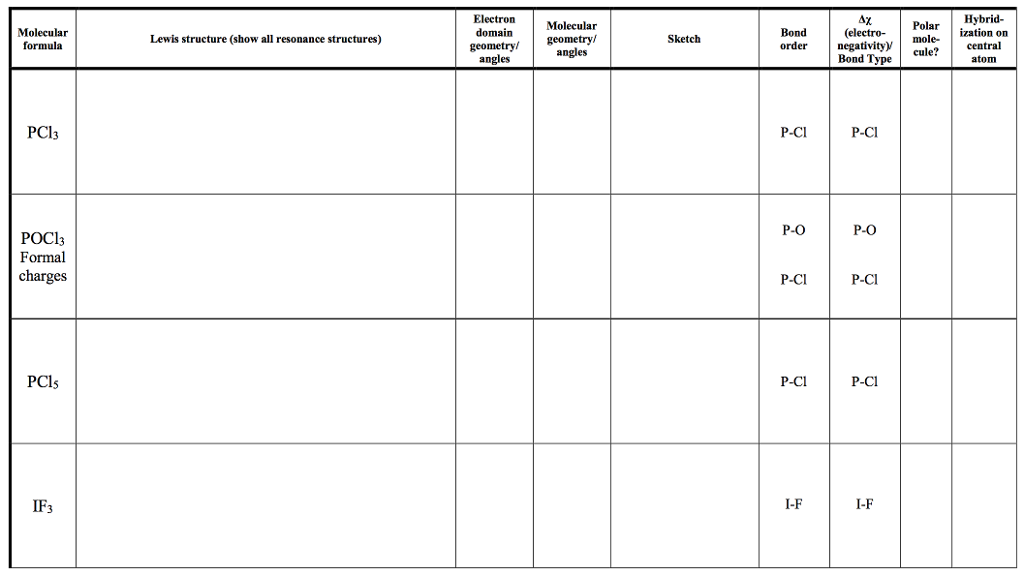
Solved Electron Domain Polar Mole Central Hybrid Ization Chegg Com

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube

Pcl3 Lewis Structure And Molecular Geometry Youtube

Resonance Structures Some Molecules Are Not Well Described By Lewis Structures Typically Structures With Multiple Bonds Can Have Similar Structures With Ppt Download

Simple Bonding Theory Review Of Lewis Structures And Vsepr Theory Slideshow And Powerpoint Viewer Resonance Some Molecules May Have More Than One Valid Lewis Structure These Structures Differ In
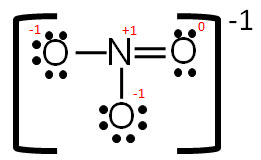
Covalent Bonding Electron Dot Diagrams Texas Gateway

Diagram Nf3 Lewis Diagram Full Version Hd Quality Lewis Diagram Orquestralivre Arcieriarcobaleno It
Fresininda

Simple Bonding Theory Review Of Lewis Structures And Vsepr Theory Slideshow And Powerpoint Viewer Resonance Some Molecules May Have More Than One Valid Lewis Structure These Structures Differ In
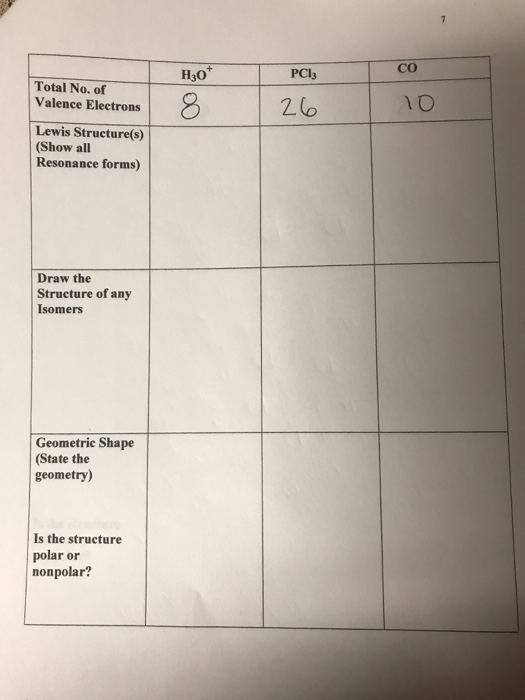
Solved Co H30 Pcl3 Total No Of Valence Electrons Lewis S Chegg Com

Chapter 10 Flashcards Quizlet
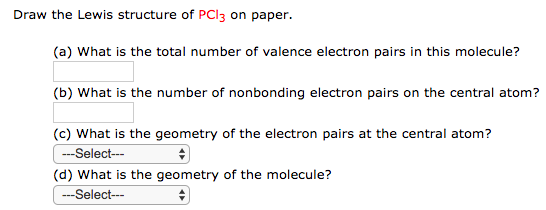
Solved Draw The Lewis Structure Of Pcl3 On Paper A Wha Chegg Com

Chapter 9 Ionic And Covalent Bonding 1 In Which Pair Do Both

Simple Bonding Theory Review Of Lewis Structures And Vsepr Theory Slideshow And Powerpoint Viewer Resonance Some Molecules May Have More Than One Valid Lewis Structure These Structures Differ In

Formal Charges And Resonance Chemistry For Majors
Dot Diagrams Kaiserscience
1
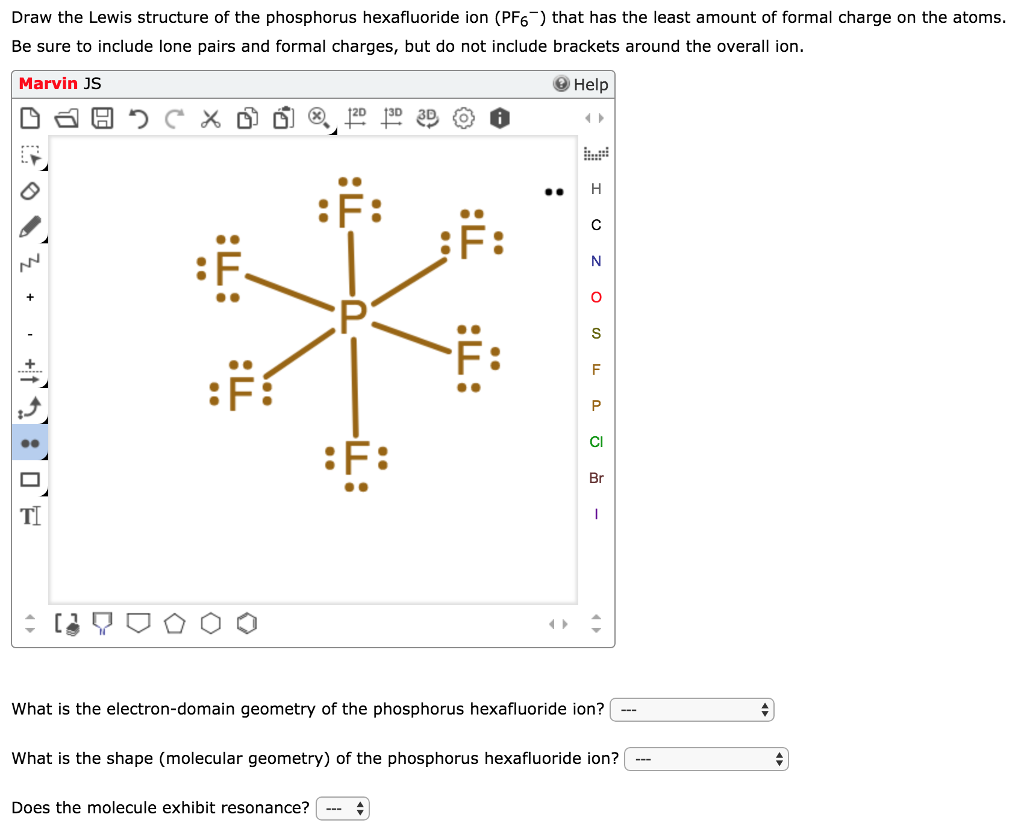
Diagram Lewis Dot Diagram Of Phosphorus Full Version Hd Quality Of Phosphorus Silverfusa110 Fujiya It
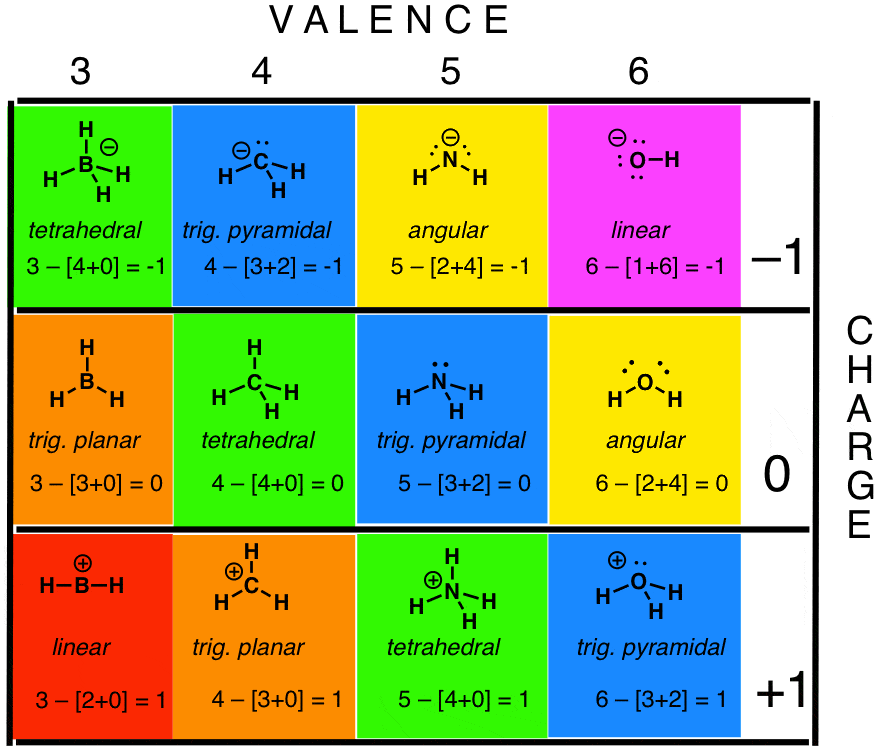
How To Calculate Formal Charge

Chem Filling In The Valence Electrons Of An Electron Dot Structure Lewis Structure Scientific Tutor
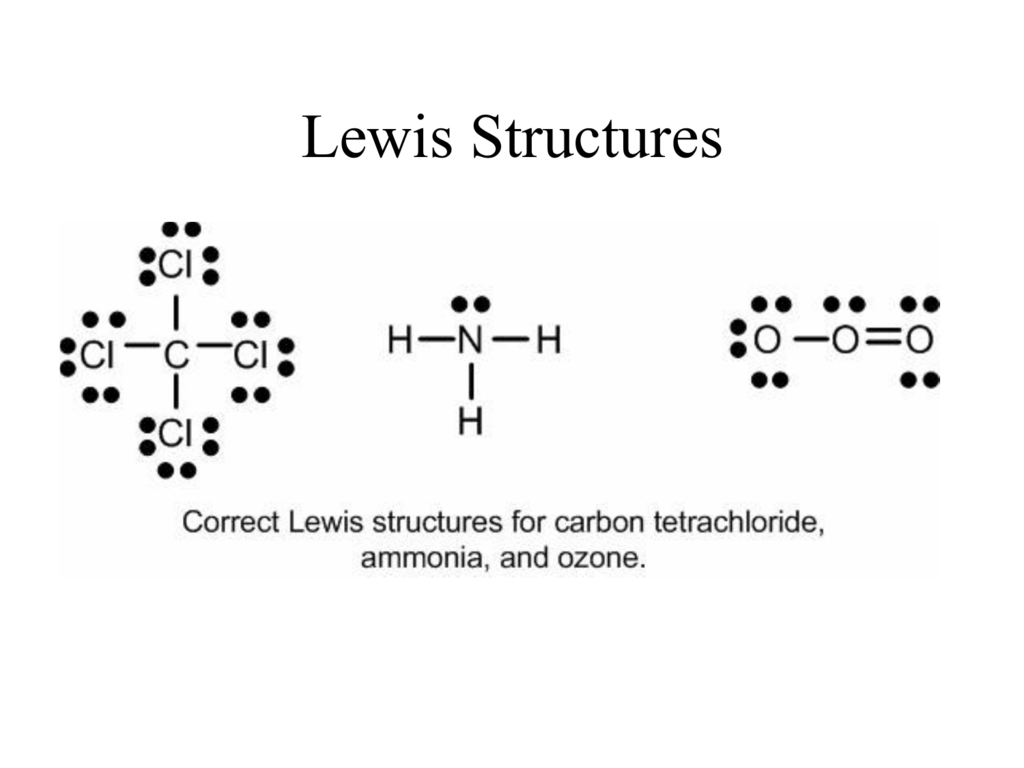
Lewis Structures
Www Mctcteach Org Chemistry C10 C10 Handouts Molecular modeling v 8 18 Pdf

The Lewis Structures Of Four Compounds Are Given Oss T Ocso C Which Of These Molecules Are Polar Pc13 Co2 So2 Ch Homeworklib

Chapter 10 Problems Key Chemical Polarity Covalent Bond
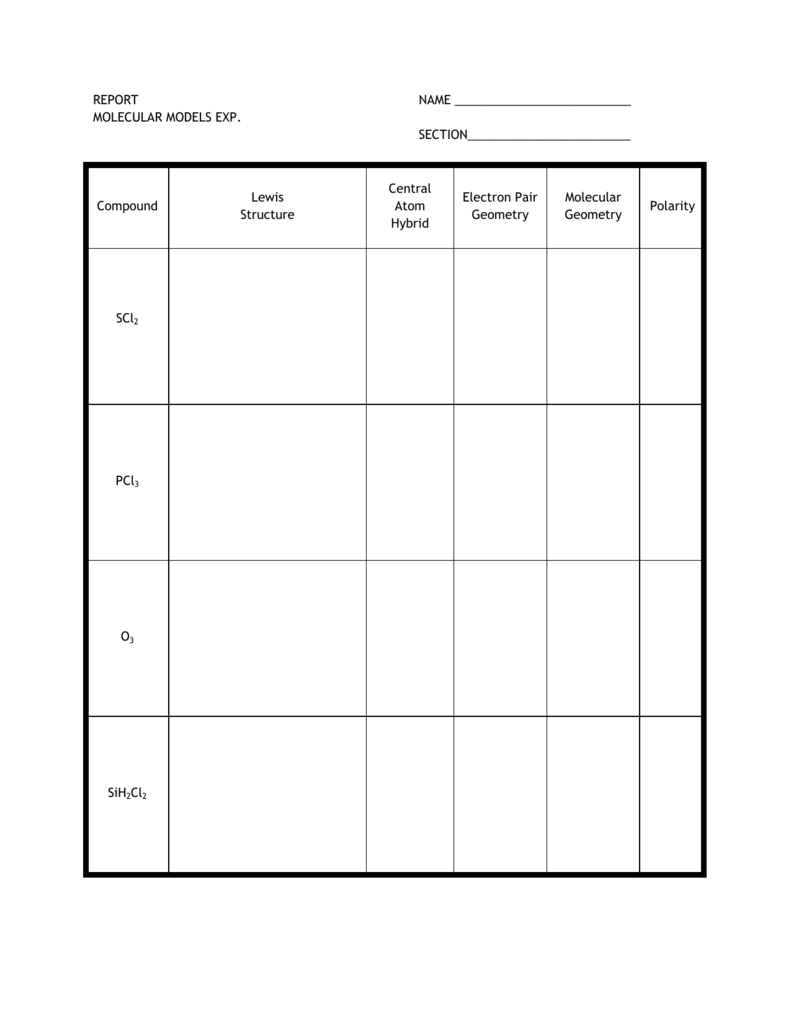
Report Name Molecular Models Exp Ars

Pcl3 Lewis Structure How To Draw The Lewis Structure For Pcl3 Phosphorus Trichloride Youtube

Covalent Bonds 1 Bonds Formed By Sharing Electrons Between Atoms 2 Group Of Atoms Held Together By Covalent Bond Called Molecule Octet Rule 1 8 Electrons 4 Pair Around Atom Hydrogen 2 E

Chapter 10 Flashcards Quizlet

Draw A Second Resonance Structure For The Following Radical Shown Below Study Com

The Lewis Structures Of Four Compounds Are Given Oss T Ocso C Which Of These Molecules Are Polar Pc13 Co2 So2 Ch Homeworklib
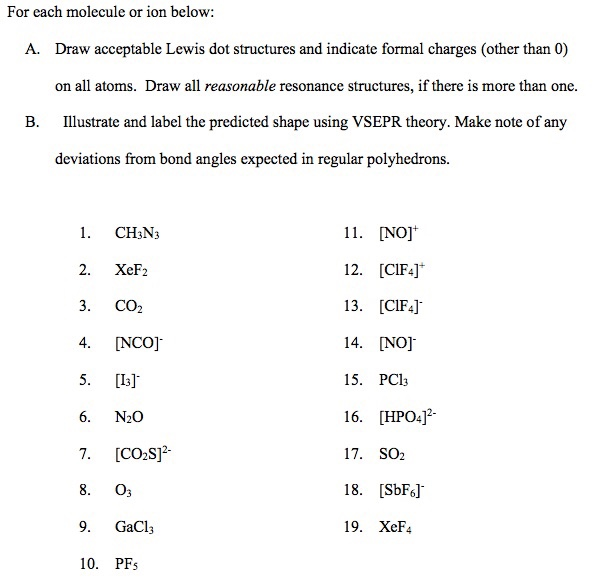
Solved For Each Molecule Or Ion Below Draw Acceptable Lew Chegg Com

Ch 9 Molecular Shape Bonding Patterns Polarity Dipole Ppt Download
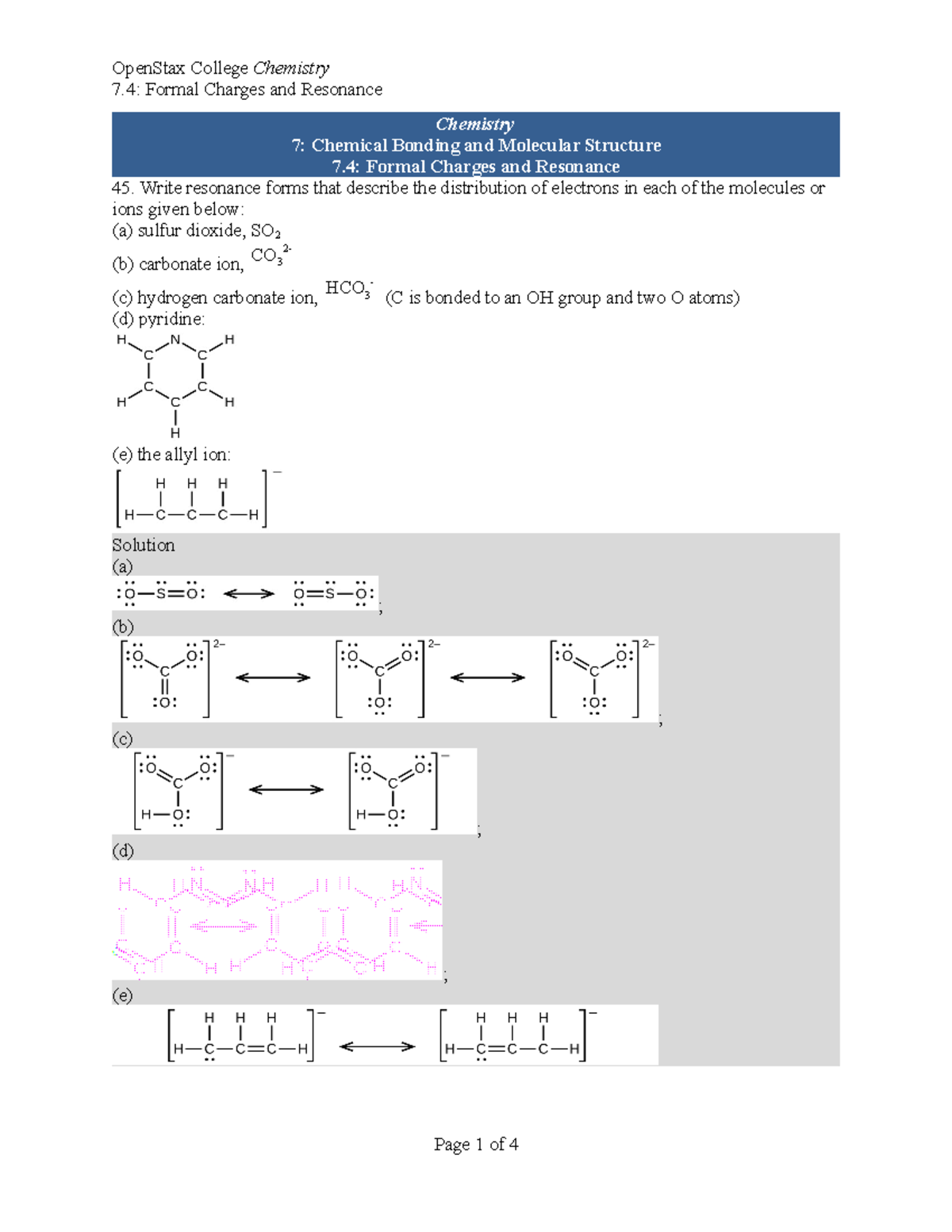
Cnx Chemistry Ssm Ch07 Mod04 Sci105 Usc Studocu

Chapter 8 Concepts Of Chemical Bonding

Draw The Resonance Structures For Ch 2 Nn Calculate The Formal Charges On Each Structure And Circle The More Stable Resonance Structure Study Com

Polarity Lewis Structures And Resonance Powerpoint Presentation Free Online Download Ppt 9ercbc



