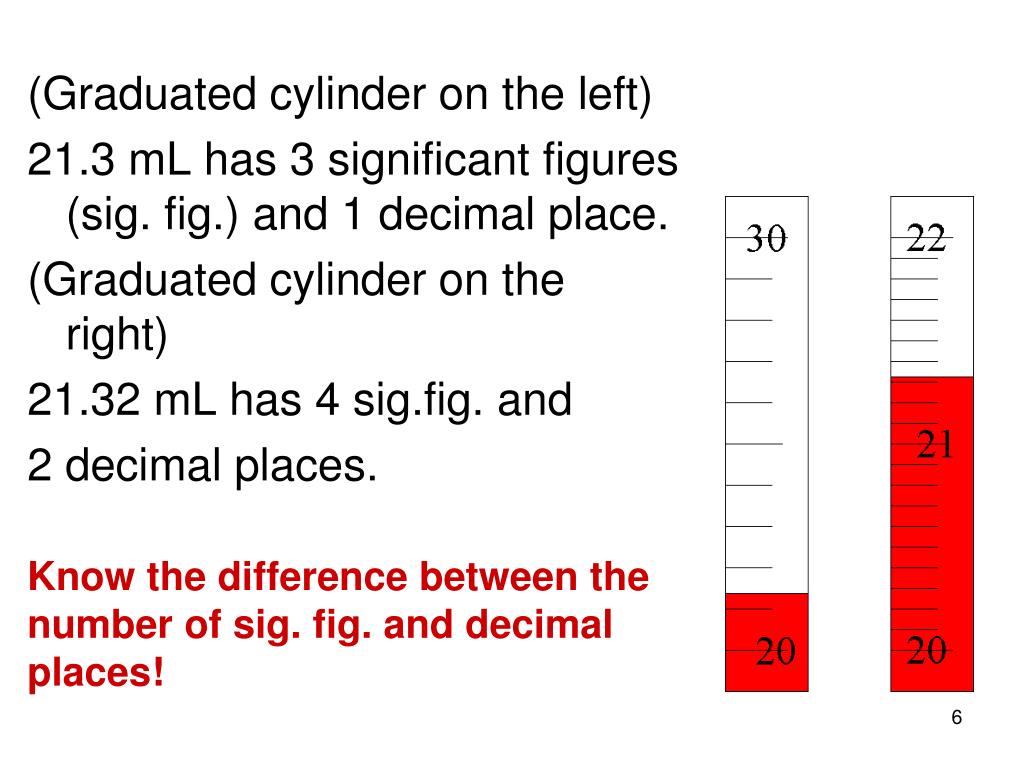
25 Ml Graduated Cylinder Decimal Places

Reading A Graduated Cylinder Teaching Math Graduated Cylinders Learning Math

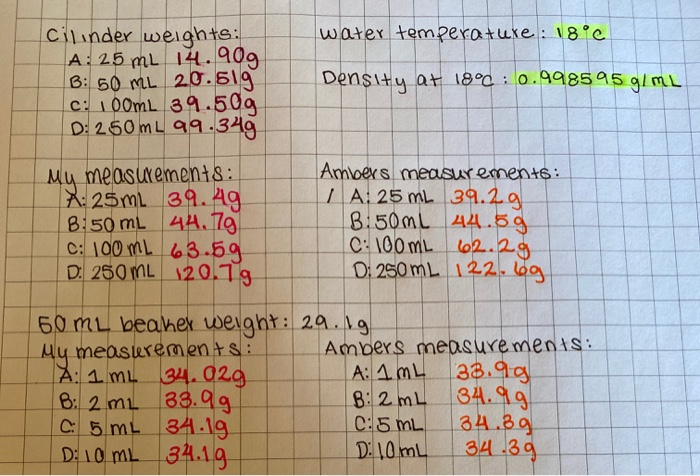
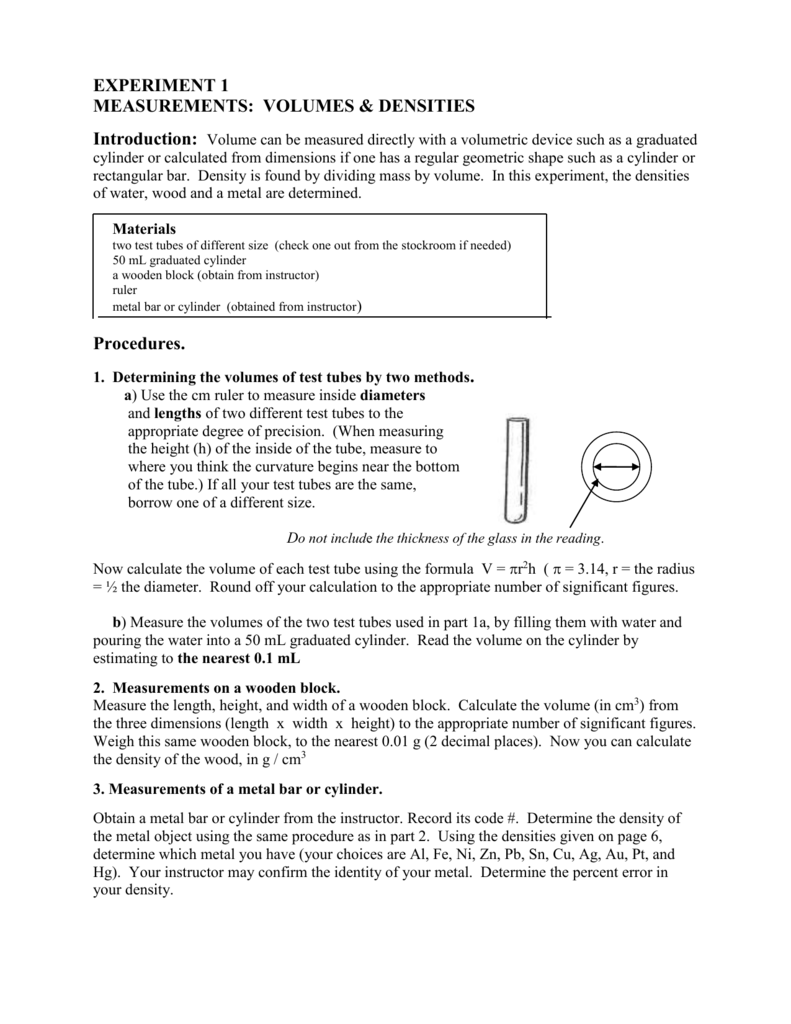
Lab 1

Solved Measuring Mass And Density Report Name Date Sectio Chegg Com

Reading Scales Section 1 3 Our Metric Rulers Are Marked Off In Centimeters 10 Centimeters Are In One Decimeter Ppt Download

Measurement Uncertainty Accuracy And Precision Ns 104 General Chemistry 1 Svc Openstax Cnx
Significant Figures
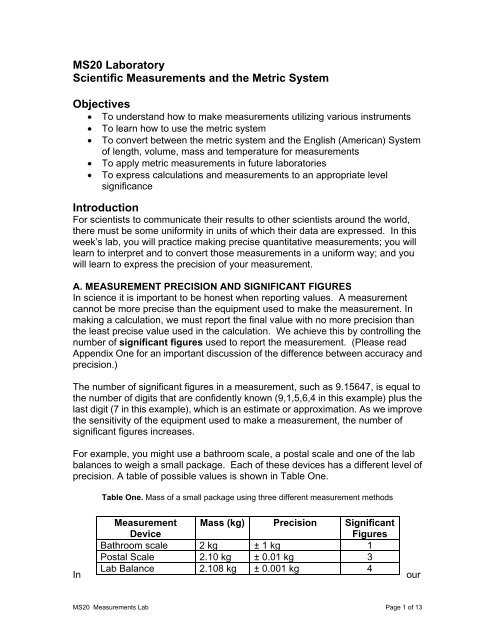
Therefore, the scale increment is 5 mL/10 graduations = 0.5 mL/graduation.

25 ml graduated cylinder decimal places. In general measurements made with graduated cylinders can be estimated one decimal place beyond the smallest division. Burets with liquid capacities of 25.00 mL and 10.00 mL are also available. Trailing zeros are significant only if the decimal point is specified.
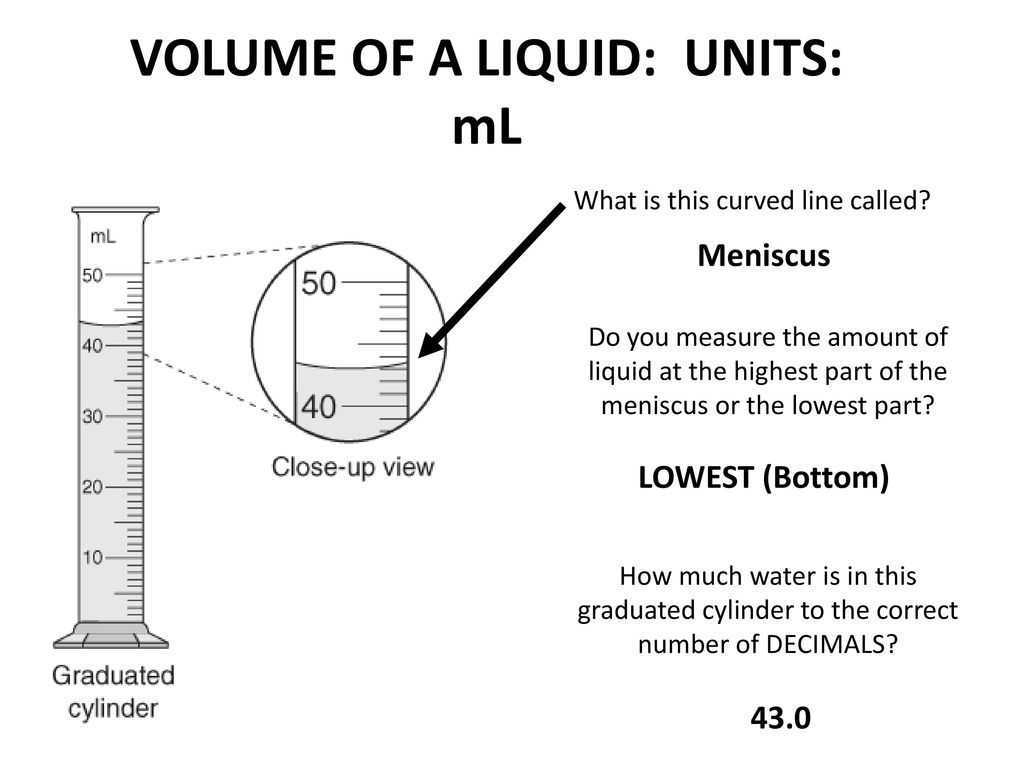
This curve is called the meniscus. An empty graduated cylinder weighs .450 g. Are the two measured masses consistent to the centigram place (i.e.
When measuring liquid volumes, the graduated scale must be read from the lowest point of the curved surface of the liquid – the liquid meniscus. A buret is used to deliver a measured amount of liquid into a container. Measure out 50 mL of DI water with a 50-mL graduated cylinder.
Next, count that ten intervals are between the labeled graduations. Wash and scrub the cylinders to. If you look at a 10mL graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1mL).
Each of these shorter lines indicates 0.2 mL. Volumes measured with a 100 mL graduated cylinder that has a scale increment of 1 mL should be reported to ___ decimal place(s). Uncertainty in Measurement of a Volumetric Pipet 1.
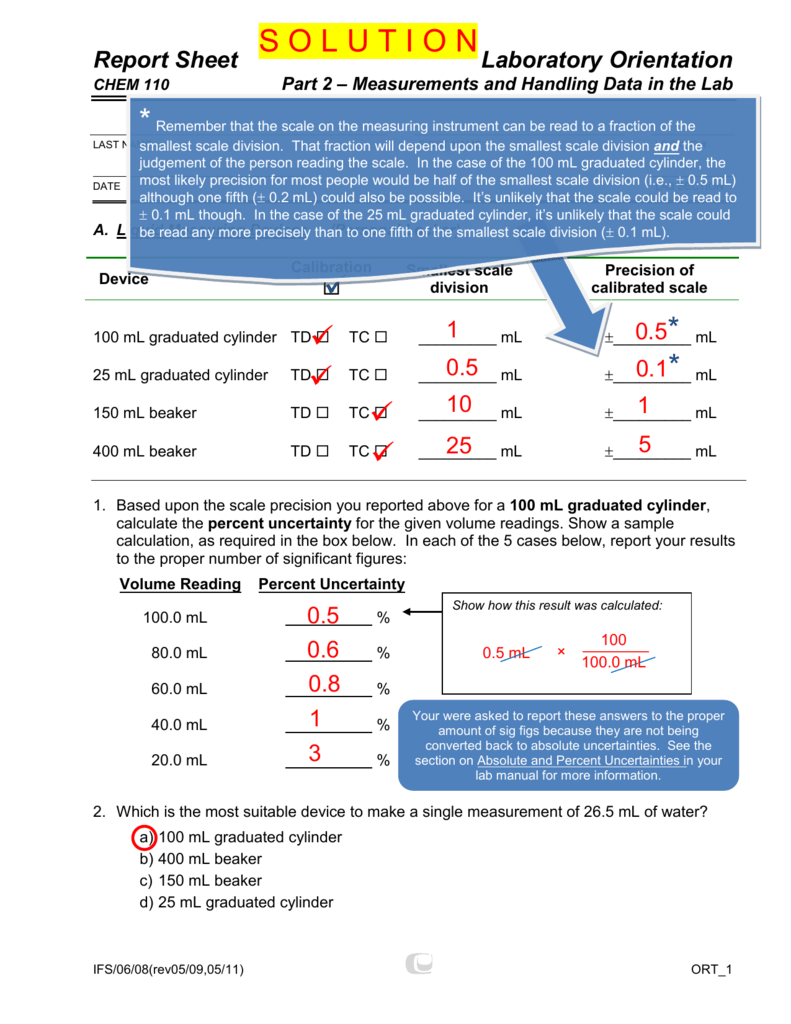
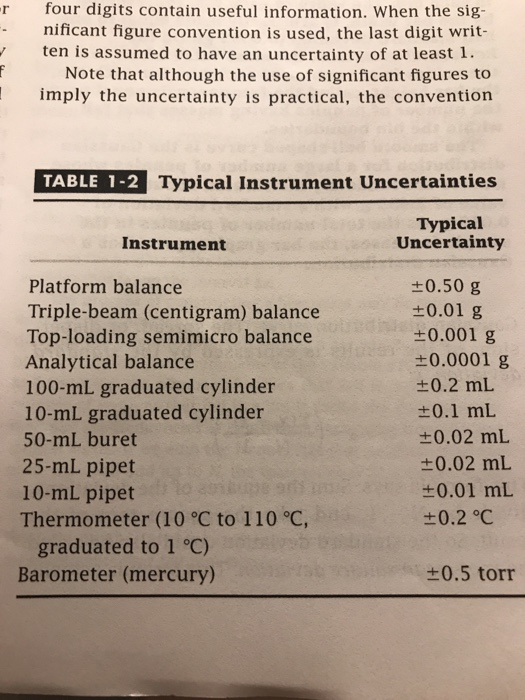
+/- 0.5 mL (always record to 1 decimal place). +/- 0.1 mL (always record to 1 demical places) 100 mL graduated cylinder:. The 10-mL graduated cylinder scale is read to the nearest 0.01 mL and the 500-mL graduated cylinder scale is read to the nearest milliliter (1 mL).
Notice that we can read volumes to the tenth of a milliliter using a 50 ml. Why can’t we repeat this procedure with the 250 mL beaker?. Write the volume using the correct number of decimal places and units.
Answer In the 25-mL graduated cylinder, first subtract 25 mL - mL = 5 mL. Whenever instructions refer to a “column” of data, you can find the column number at the bottom of the appropriate table. This is called a meniscus, and it is to be read at its lowest point.
We should round the result to the same number of decimal places as the number with the least number of. A buret is a scaled cylindrical tube attached to a stopcock, or valve. When filled to 50.0 mL with a liquid it weighs 110.810 g, what is the density of the.
Recording the volume 2 decimal places means that you are measuring, or rounding your measurement. That means when you read the volume, you can estimate to the hundredths place (0.01mL). Portion of a 10mL graduated cylinder Use the bottom of the meniscus to determine the volume in the 10mL graduated cylinder.
These should include the equations. A solid object weighing 73.5 g was immersed in the liquid, raising the liquid level to 43.9 mL. For example, if the level of the liquid were halfway between the 24 and the 25 ml.
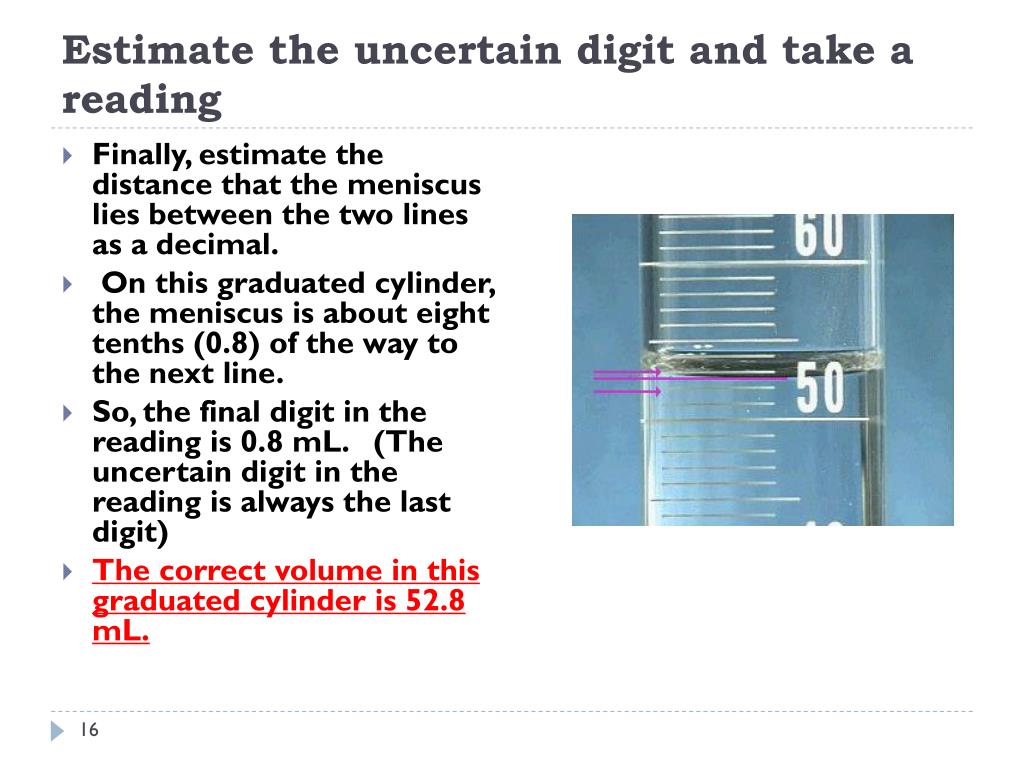
The balance should always be zeroed before weighing and you should always. To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 mL marks into tenths of a milliliter, and then make a reading (estimate) at the bottom of the meniscus. In general, numerical scales such as the one on this graduated cylinder will permit measurements to one-tenth of the smallest scale division.
Sig figs 1 100 mL graduated cylinder colored water 1 decimal place mL 2 25 mL graduated cylinder colored water mL 3 50 mL beaker colored water mL 4 Electronic balance “A” block g 5 Electronic balance “B” block g 6 Alcohol thermometer water °C 7 Temperature. Change the number to scientific notation. Since your buret is graduated to 0.1 mL, you will read your buret to 0.01 ml.
This rubber stopper was placed in the graduated cylinder containing 25.0 mL of liquid water. Mark, the reading is estimated at 24.5 mL. Graduated cylinder (one decimal place).
Depends on the precision of your balance. For a standard 10 mL graduated cylinder, you can't measure with a greater precision than 1 mL (no decimals). In this case, the measurement interval marked by each line equals 1 (the numbered interval) divided by 5 (the count from one interval line to next), or 1 ÷ 5 = 0.2 ml.
Ion Measuring Device Object # of Decimal Places Tool Can Report Reading # of. Record the weigh on your data page making sure to write down all four decimal places. All volumes MUST be recorded to 1 decimal place (0.1 mL).
Number of significant figures for 75 mL in a 250 mL and 125 mL Erlenmeyer flask. Therefore the buret actually will hold more than 50.00 mL of solution. Density (Attach sample calculations of each type necessary to obtain the results in the table below.
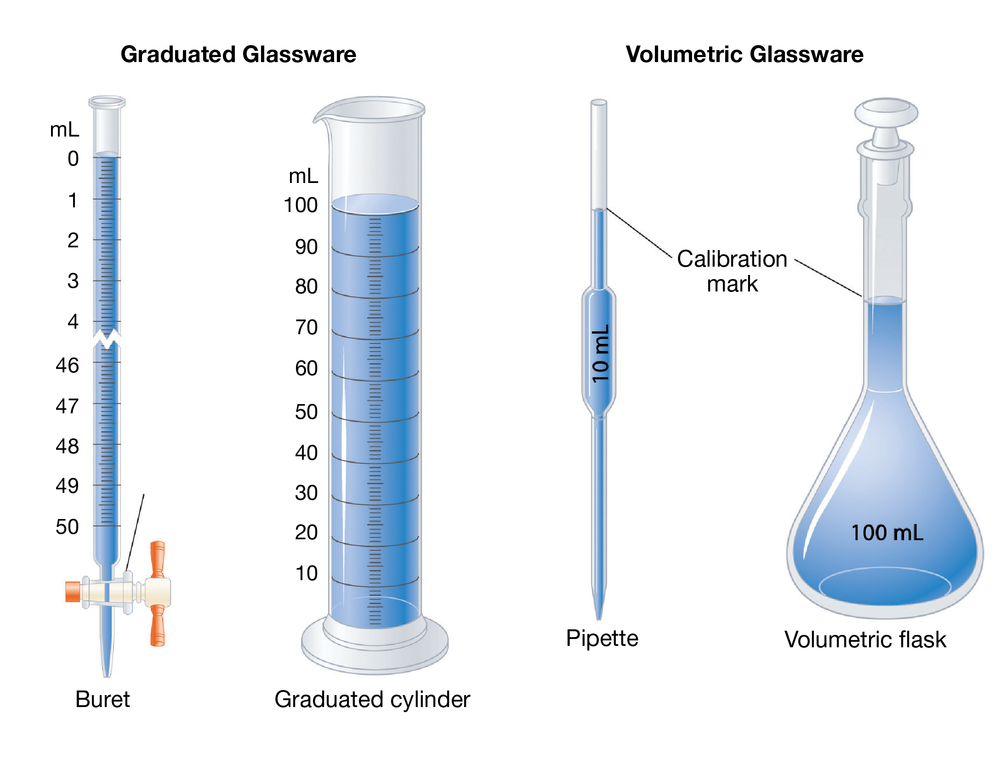
A buret is designed to. Remember to read the volume at the bottom of the meniscus. Glass is easier to clean, but more fragile and expensive than plastic.
Always use the smallest graduated cylinder that will hold the entire volume. Traditionally, 2 decimal places should be used to record the volume of a 10 ml volumetric flask. It is given that the graduated cylinder of volume 25 ml has a major scale marked every 2 ml and minor scale with 0.2 ml.
Read the exact volume at the bottom of the meniscus and record it in Table 1. It is graduated in 0.1 mL increments, with the 0.00 mL mark at the top and the 50.00 mL mark near the bottom. The smallest division of this graduated cylinder is 1 mL.
Using a grease pencil, label one 25 mL graduated cylinder “A” (for Alcohol) and a second cylinder “W” (for Water). A 100 mL grad cylinder, to 1 mL (no decimals) a 10 mL pipet can be used to 1 decimal place. So, this sample graduated cylinder measures accurately to 0.2 ml.
The scale in this case has 1-mL divisions, and so volumes may be measured to the nearest 0.1 mL. 5.50 mL) and the 100-mL graduated cylinders are always read to 1 decimal place (e.g. As well, beakers are usually not graduated precisely.
You will estimate the last decimal place. Round to the tens place - set by 550) (3) 0.03 (1 significant figure - round to hundredths place) (4) 7.4 x 10 7 (2 significant figures - set by 1.3 x. 3 Measure 10 mL of water in the small beaker provided, then transfer the water to the 10 mL graduated cylinder (smallest one).
Estimated digit place value and number of decimal places in reported value for. The 10-mL graduated cylinders are always read to 2 decimal places (e.g. Measure the volume of water in each.
Graduated cylinders are manufactured in sizes ranging from 5 mL to 00 mL. Given investigation quantitative data, students will determine its degree of precision and/or accuracy and causes for uncertainties in measured data. The first 2 digits 30.0 are known exactly.
Reasonable estimation can be made if the measured liquid lies between the marked intervals, but this estimated. Notice that the marks do not go all the way to the stopcock. (1 decimal if you're using a Class A cylinder).
50 mL graduated cylinder Since the volume of the liquid has a one decimal place, the glassware to be used should be able to measure a volume to the nearest 0.1mL. The water level rises to the 49.10 mL mark, from this information, calculate the density of iron. Hundredths place) when rounding the milligram balance reading to two decimal places?.
Thus, even if you very very carefully fill the beaker to exactly the $15.5~\mathrm{mL}$ line with liquid, there's a very real chance that the line doesn't actually correspond exactly to a $\mathit{15.5~mL}$ fill volume. When water is placed in a glass cylinder, a concave surface forms;. The last digit 30.0 is uncertain.
10 mL graduated cylinders let you measure volumes up to 10.00 mL to the nearest 0.01 mL. With a 50-mL graduated cylinder, read and record the volume to the nearest 0.1 mL. A graduated cylinder was filled with 25.0 mL of liquid.
There is a line half way around the cylinder to indicate the even number of mL quantities. You will be using a 25 mL buret with graduations every 0.1 mL. It sank to the bottom of the cylinder, and the volume reading changed to 31.2 mL.
An unknown metal sample weighs 18.1 g. +/- 0.02 mL (always record to 2 decimal places) 25 mL graduated cylinder:. In reading numbers from a graduated scale, you always interpolate between the graduation marks.
The correct reading is 30.0 mL. In this case, the volume of the liquid can be read to the nearest 0.1 division. This measurement is reported with 3 significant figures and the volume would be 8.68 mL.
Transfer the contents of the graduated cylinder to the beaker or. Using a 10 ML graduated cylinder you can read 2 decimal places. 25 mL graduated cylinder.
3 Name:_____ Partner:_____ Check your work above to be sure that you reported the correct number of decimal places in your measurements and that your final volume answers have the correct number of significant figures. This is also measuring volume. 1 Perform the following mathematical operation and report the answer to the proper number of significant figures and units:.
25 mL graduated cylinders let you measure volumes up to 25.0 mL to the nearest 0.05 or 0.02 mL, depending on your exact cylinder. Using a 25 mL graduated cylinder, obtain the volume of your unknown solid by displacement of water. At the front bench, you will find a burette, 10-mL graduated cylinder, 100-mL graduated cylinder and 100-mL beaker, each filled with a certain quantity of water.
Record the mass of a clean and dry 50-mL beaker. Thus, a 10 mL graduated cylinder will be accurate to within 0.1 mL. 10 mL graduated cylinder:.
Estimate volumes to the nearest 1/10th between graduations. 25 mL graduated cylinder This graduated cylinder has white lines going completely around the cylinder to indicate odd number of mL quantities. Volume in graduated cylinders:.
We should round the result to the same number of decimal places as the number with the least number of decimal. 28.5 g of iron is added to a graduated cylinder containing 45.50 mL of water. There are 5 shorter lines between each whole number.
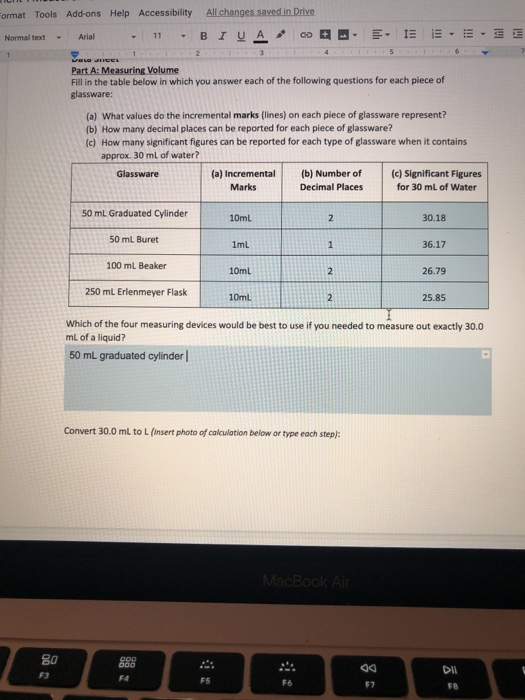
Number of Decimal Places to be Recorded Ruler (in cm) 10-mL Graduated Cylinder 50-mL Graduated Cylinder 50-mL Buret 250-mL Beaker Part II:. As with beakers and flasks, graduated cylinders are available in either glass or plastic;. What is the density of this metal (in g/mL using 2 decimal places)?.
The graduated cylinder markings are every 1-milliliter. You will need to obtain an initial volume of water and then gently place the cylinder in the water.

Graduated Cylinder Wikipedia
1 Calculate And Record The Mass Of Water In Each Chegg Com
Lab Investigation 1 What Is The Best Practice For Laboratory Measurement
Solved Part C Determining The Density Identity Of An U Chegg Com
1 9 Essential Skills 1 General Chemistry

Measurement Texas Gateway

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry

Unit 1 Worksheets

Significant Figures Lab Middlebury College Chem 103 Lab

1 2 Uncertainty And A Graduated Cylinder Youtube

Chemistry Tutorial 2 Flashcards Quizlet
Reading The Volume From A 10 Ml Graduated Cylinder

Determine The Density Of The Cube Step 1 Use The Chegg Com

Chem 0010 Online

How Do We Read Our Measurements In Science Significant Figures All Numbers In A Measurement That Are Definitely Correct Plus One Estimated One Special Ppt Download

How To Read A Graduated Cylinder Youtube

Lab 1

Solved Ii Volume Of A Liquid 1 Examine The Four Graduat Chegg Com
Significant Figures

Earth Science Making Measurements And Reading Scales Ppt Download
Ppt Review Of Significant Figures Powerpoint Presentation Free Download Id
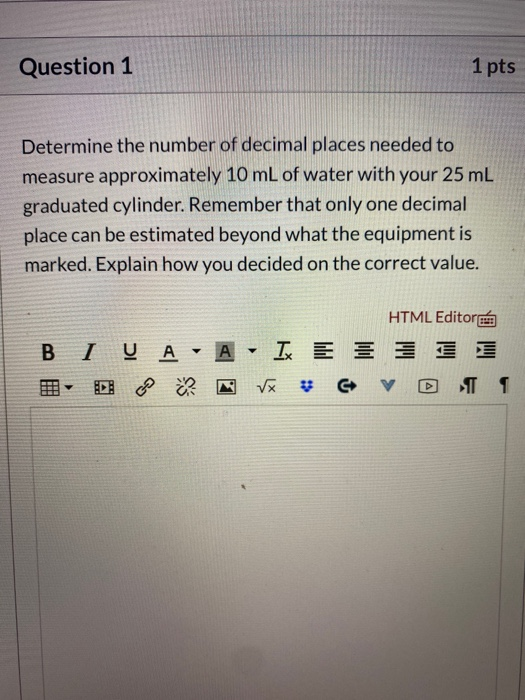
Solved Question 1 1 Pts Determine The Number Of Decimal P Chegg Com

Solved 6 Which Of The Following Is Consistent With The Chegg Com

Lab Practical Part 2 Flashcards Quizlet
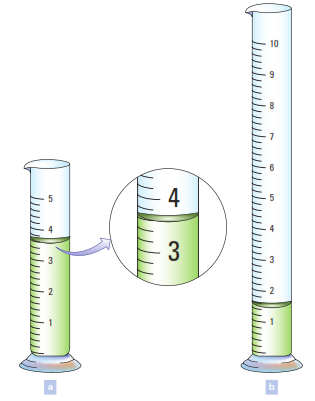
State The Volume Of Liquid In Each Graduated Cylinder In The Figure Below And Explain How You Decided Upon The Appropriate Number Of Significant Figures The Markings Are Calibrated To Be Read
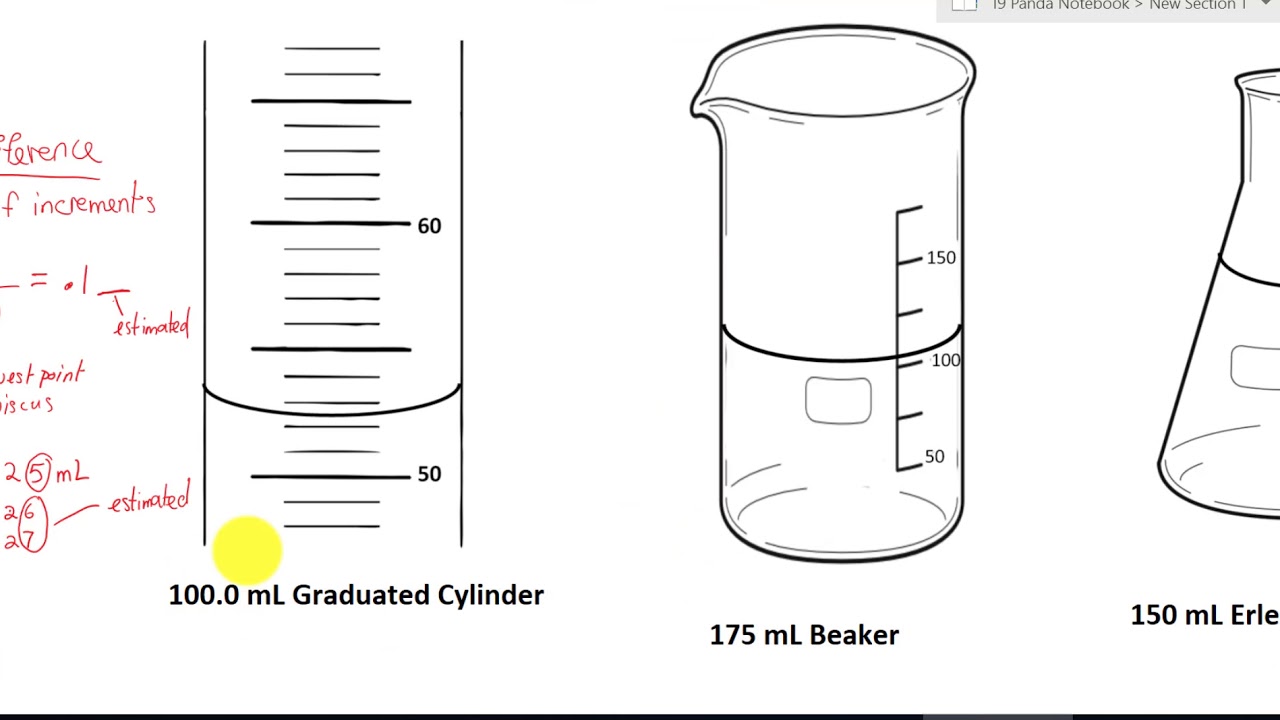
Read Measurement Of Graduated Cylinder Beaker And Flask Youtube

Making Measurements And Reading Scales Ppt Video Online Download

Significant Figures Middlebury College Chem 103 Lab

Handling Of Pipette Buret Separatory Funnnel Graduated Cylinder

Chemistry 1151 Lab Midterm Flashcards Quizlet
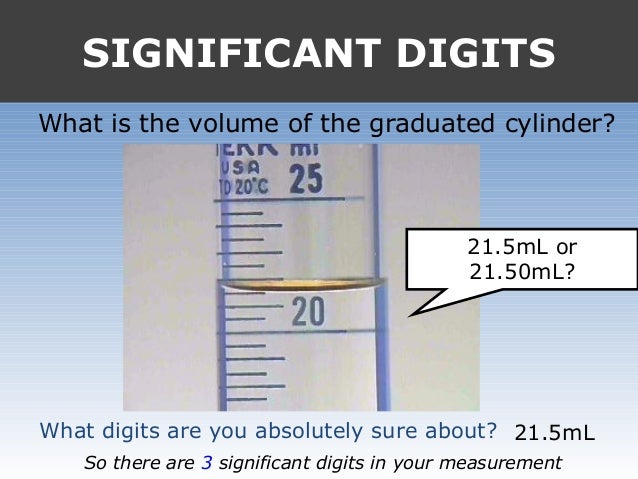
01 Significant Digits

If You Measure Water Of A Graduated Cylinder And Find It To Be 23 Would You Write It As 23 Ml Or 23 0 Ml Quora

Survey Of Chemistry Laboratory I Determination Of The Density Of Water Ppt Download
Q Tbn 3aand9gcsbhv6zhf72sv5z1ycdedywkxuuayywzbqex0qhtkq Usqp Cau

Significant Figures Uncertainty Physics Chemistry Review
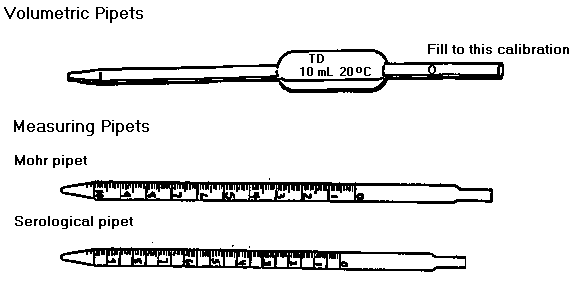
Laboratory Techniques

Accuracy And Precision Measurements Significant Figures Sig Figs Ppt Download
2
Assets Pearsonschool Com Asset Mgr Current 1731 Lumina 4c M02 Timb8046 05 Se2 Pdf

Solved Ii Graduated Cylinders Cylinder Size 10 Ml 25 Ml Chegg Com
Laboratory Techniques And Measurements

If You Measure Water Of A Graduated Cylinder And Find It To Be 23 Would You Write It As 23 Ml Or 23 0 Ml Quora

Density Lab Teacherweb

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry

Graduated Cylinder Wikipedia
Sd34science Files Wordpress Com 15 09 Precision And Uncertainties For Common Lab Equipment Lab 1 Pdf

Answered Experiment 1 Measurement Ert Friday Bartleby
Q Tbn 3aand9gcrlnhrxfmi7jtnssas74avqglzp2me6hsyqwuvqo7m Usqp Cau

Ap Chem Volumetric Glassware Lab Write Up Mathematics Nature

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry

Chm1025c Chm 1032c Density Lab

Measurement And Significant Figures Youtube

Chemistry 103 Measurement Of Density
Sd34science Files Wordpress Com 15 09 Precision And Uncertainties For Common Lab Equipment Lab 1 Pdf
Pubs Rsc Org En Content Getauthorversionpdf C4rpa

Solved Circle The Correct Response Or Fill In The Appropr Chegg Com

Lab 1
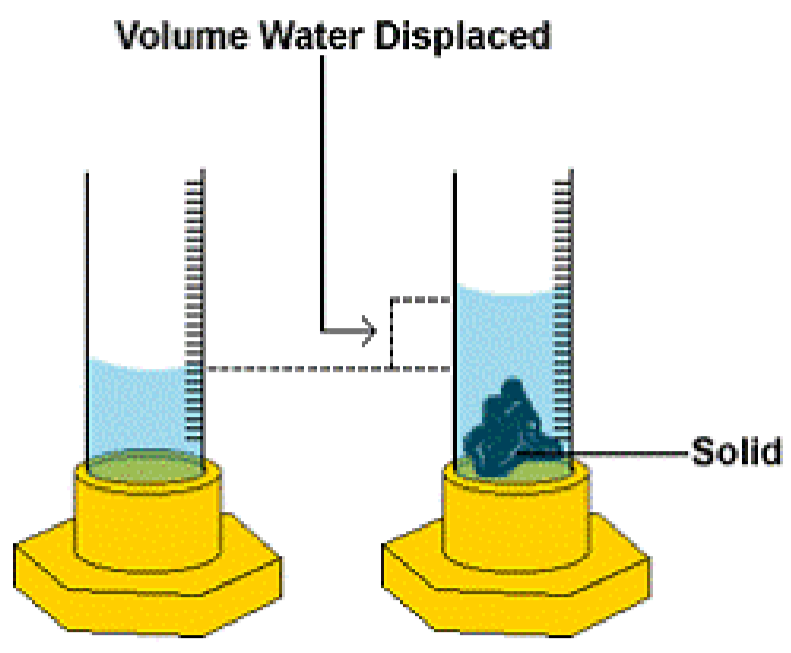
1 Measurements In The Laboratory Experiment Chemistry Libretexts
Lab Investigation 1 What Is The Best Practice For Laboratory Measurement
Q Tbn 3aand9gcrq9ii Jj9gidjsp9be0ptxqo0f14tf15z0odto2nnpjooigsxe Usqp Cau
Scientific Measurements

I L Beaker 2 5 3 0 Cm Piece Of Magnesium 15 Cm Cop Chegg Com
Pdf Document Capuchem Ca Capu Logo

Accuracy And Precision

Solved Ii Graduated Cylinders Cylinder Size 10 Ml 25 Ml Chegg Com

Lab 1

Solved T Laboratory Questions You Performed A Calculatio Chegg Com
Web Gccaz Edu Rob Safety density Density Bkgd Pdf

Solved If 25 3 Ml Of 1 0 M Naoh Are Measured In A 100 Ml Chegg Com
Ppt Honors Chemistry Powerpoint Presentation Free Download Id

Lab Overview Ppt Video Online Download
Q Tbn 3aand9gcr18zsswwci6alk7xewmhsmkvx3lm Fnag24m Yggy Usqp Cau
Www Michigan Gov Documents Mdch Measures Power Point 7 Pdf
Reading The Volume From A 25 Ml Graduated Cylinder

With Ground In Thermometer Precisely Adjusted Up To Three Decimal Places
Reading The Volume From A 100 Ml Graduated Cylinder

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry
36d5l25ig13rrnnc3w4af9 Wpengine Netdna Ssl Com Wp Content Uploads Sites 140 14 06 161lab1scientificmeasurements Updatedndpsgf Pdf
Laboratory Techniques And Measurements

Answered S Take By Your Lab Ulat Is The Bartleby

The Density And Volume Of A Water Manualzz
Http Faculty Sites Uci Edu Chem1l Files 13 11 Rdgvolflaskpipet Pdf

2 4 Significant Figures Chemistry Libretexts

Significant Figures Chemclass Ol Org

Metric Measurement Lab
Solved 6 The Density Of Water Is Determined By Measuring Chegg Com
Accuracy And Precision Measurements Significant Figures Sig Figs Ppt Download
Significant Figures

Introduction To Measurement

Survey Of Chemistry Laboratory I Determination Of The Density Of Water Ppt Download

Significant Figures Chemclass Ol Org

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry

Chm 101 102 Laboratory Manual Significant Figures And Density General Chemistry 101 102 Laboratory Manual University Of North Carolina At Wilmington Ppt Download

Measuring Cylinder At Thomas Scientific
Www Homeworkmarket Com Sites Default Files Qx 15 06 12 08 Image15 06 03 2 Pdf

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry
Experiment 1

Measurements Chemistry Department Minneapolis Community Technical College Intro To Chemistry Chem10 Lab Ppt Download



