25 Ml Graduated Cylinder Sig Figs

How To Read A Graduated Cylinder Youtube
36d5l25ig13rrnnc3w4af9 Wpengine Netdna Ssl Com Wp Content Uploads Sites 140 14 06 161lab1scientificmeasurements Updatedndpsgf Pdf

Practice Measuring Using Significant Figures In Person Or Virtually Chemical Education Xchange

If You Measure Water Of A Graduated Cylinder And Find It To Be 23 Would You Write It As 23 Ml Or 23 0 Ml Quora
Assets Pearsonschool Com Asset Mgr Current 1731 Lumina 4c M02 Timb8046 05 Se2 Pdf

Answered 2 Below Each Figure Give The Reading Bartleby
What is the density (g/mL) of an object that has a mass of 14.01 grams and, when placed into a graduated cylinder, causes the water level to rise from 25.2 mL to 33.6 mL?.

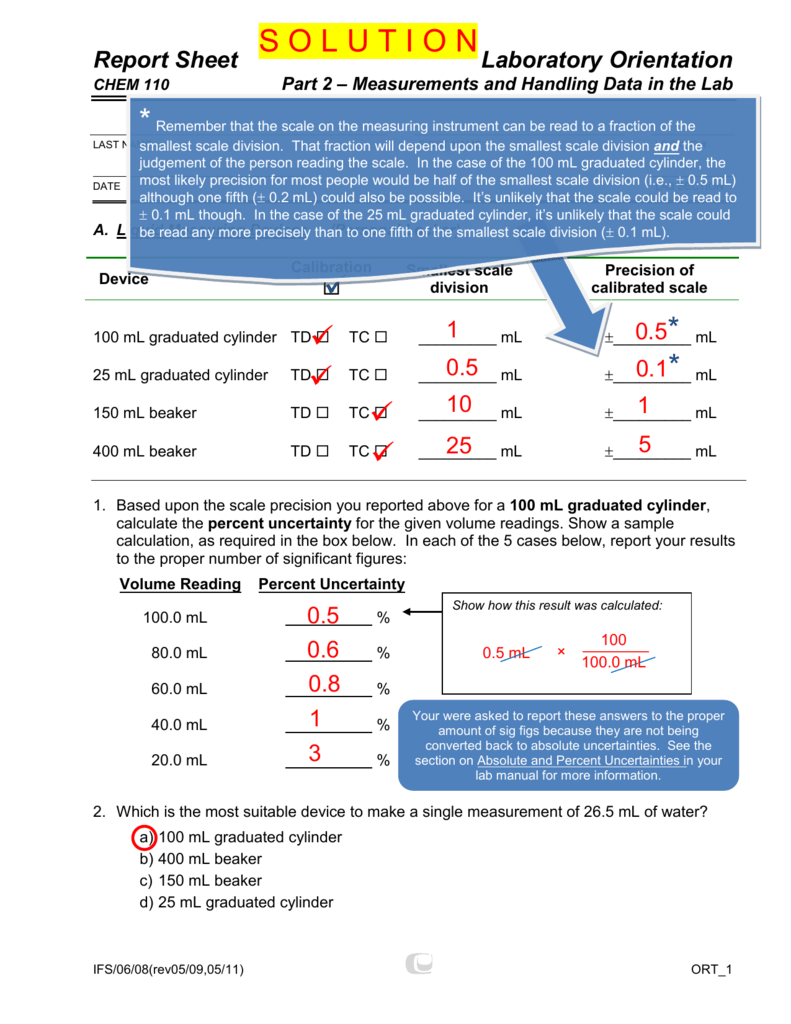
25 ml graduated cylinder sig figs. Therefore, the scale increment is 10 mL/10 graduations = 1 mL/graduation. When possible, steady the cylinder with one hand while pouring liquids in. Therefore the buret actually will hold more than 50.00 mL of solution.
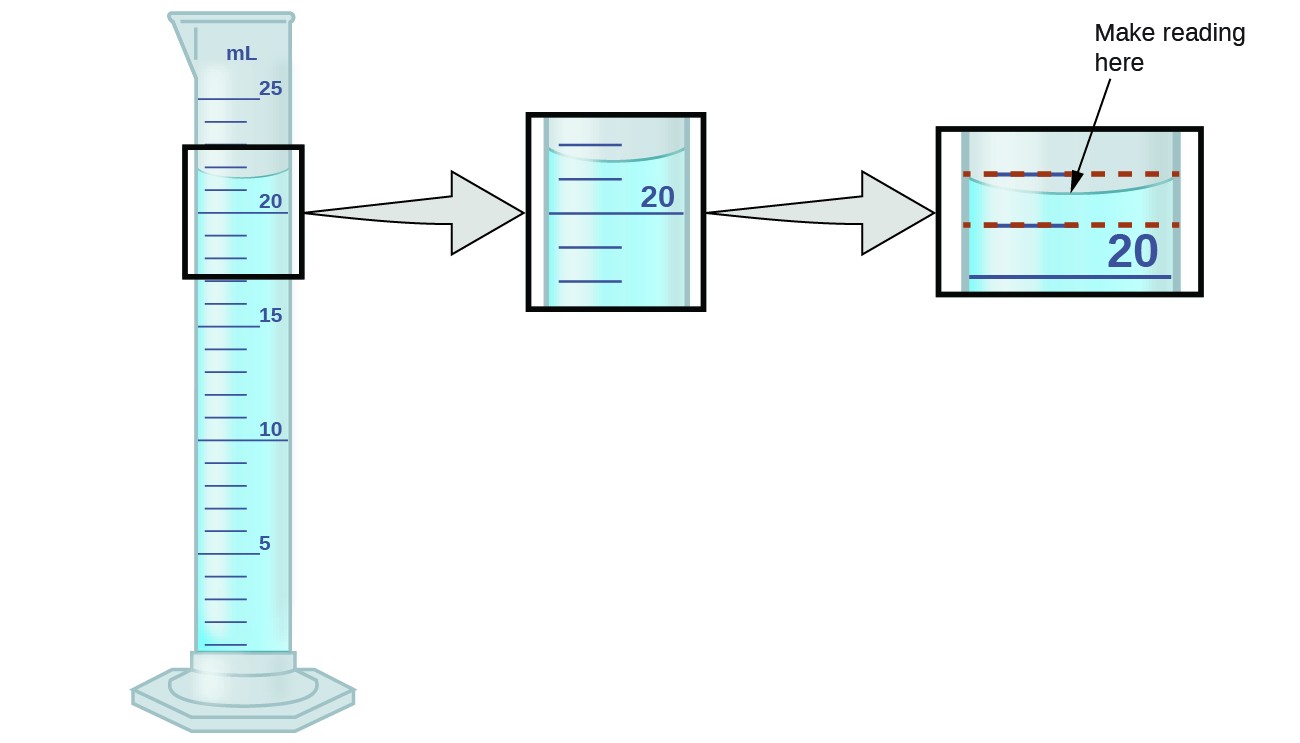
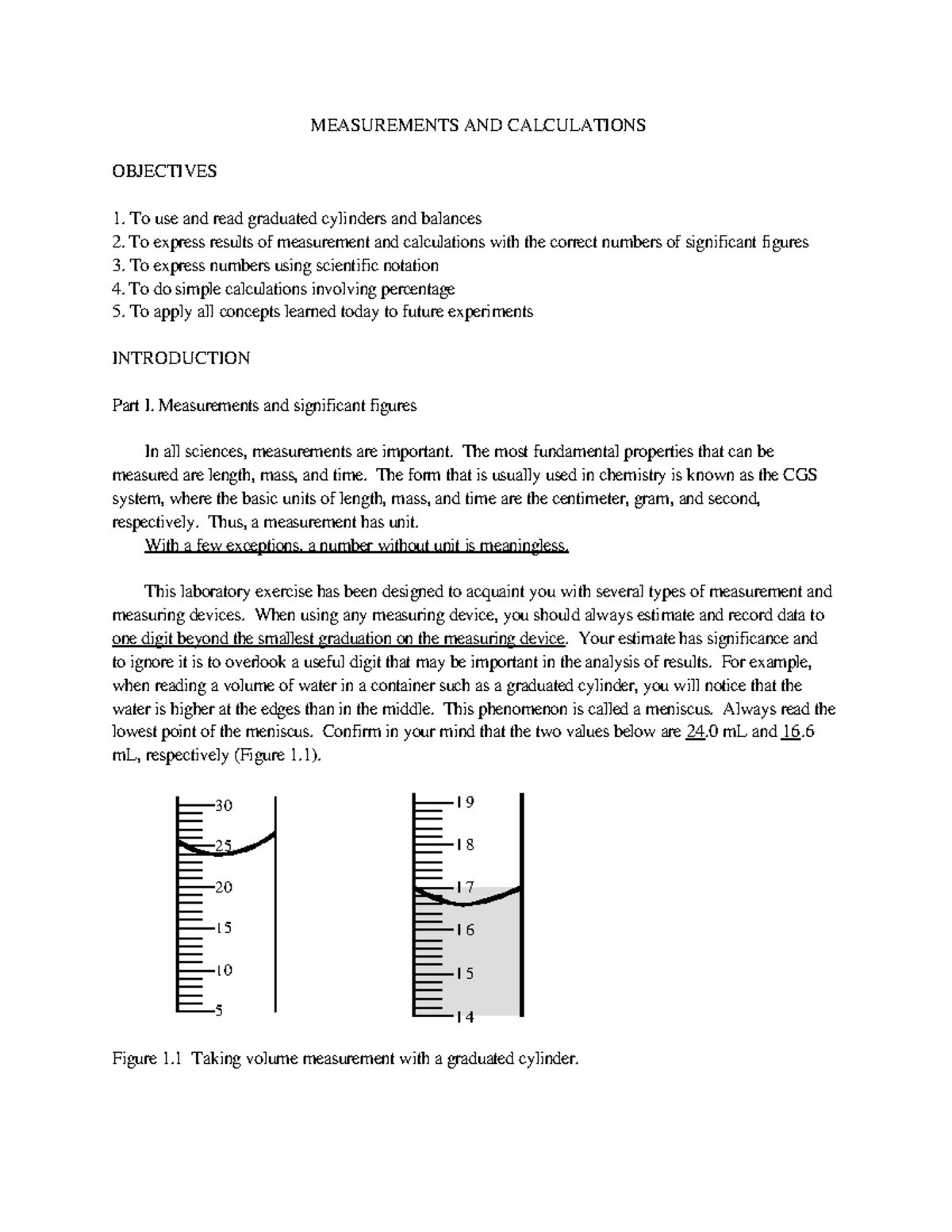
3.87mL Portion of a 10mL graduated cylinder. Using a grease pencil, label one 25 mL graduated cylinder “A” (for Alcohol) and a second cylinder “W” (for Water). To measure the volume of liquid in a graduated cylinder, you should make a reading at the bottom of the meniscus, the lowest point on the curved surface of the liquid.
The level of water rises to 18.0 mL. So, subtract 60 mL - 50 mL = 10 mL. Graduated cylinders are transparent cylinders with finely divided markings – otherwise known as graduations – marked on their side.
Cylinders for measuring up to 10 mL to have divisions at 0.1 mL, so again giving 2 sig. Density = mass / volume rearrange to:. Transfer the water from the 50-mL graduated cylinder to a clean, 100-mL graduated cylinder and again read its volume.
Notice that the marks do not go all the way to the stopcock. Transfer the water from the 25-mL graduated cylinder to a clean, 50-mL graduated cylinder and again read its volume. However you should be able to read a 10 mL graduated cylinder to within one drop, that is 0.05 mL.
Adding the measurements therefore gives 60 ml plus 3 ml plus the approximately one-third ml, or 60 + 3 + 0.3 = 63.3 ml of liquid in the graduated cylinder. 1) Determine the volume of 2.25 g of aluminum:. • Ex/ A 25 g cylinder of iron (d = 7.87g/mL) and a 1.0 gram pellet of copper (d = 8.96 g/mL) are placed in.
It is graduated in 0.1 mL increments, with the 0.00 mL mark at the top and the 50.00 mL mark near the bottom. To what volume mark will the water level rise?. Next, count that ten intervals are between the labeled graduations.
0.15 mL, 0.25 mL, 0.5 mL, 1 mL (1) 0.2 mL (15) 0.5 mL (11) 1 mL (35) 2 mL (17) 5 mL () 10 mL (18) mL (13) 25 mL (1) 50 mL (1. Density of aluminum is 2.7 g/cm3. You will estimate the last decimal place.
The correct reading for the level of the liquid in Figure 1 is 23.7 mL. Lamia hauter experiment measurements and significant figures 09/16/17 professor shashikanth. One solid rubber stopper ( either a #2, #1, or #0 as long as it freely fits into the 100 ml graduated cylinder).
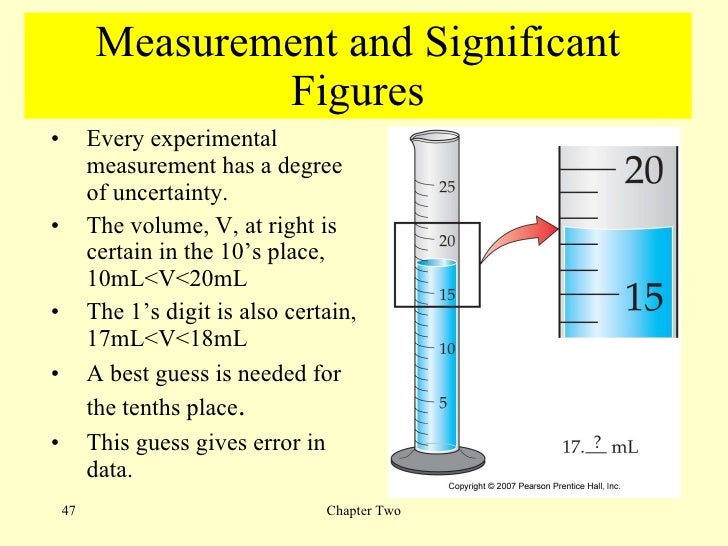
The meniscus appears to be a bit closer to the 22-mL mark than to the 21-mL mark, and so a reasonable estimate of the liquid’s volume would be 21.6 mL. Figs.) Use the bottom of the meniscus to determine the volume in the 10mL graduated cylinder. For example, if a graduated cylinder had markings for every 0.1mL, or every mL, or every L, then it is easy to tell that those measurements will be to the hundredth mL, to the tenth of a mL, and to the tenth of a L, respectively.
Suppose a sample of aluminum was placed in a 25-mL graduated cylinder containing 10.5 mL of water. Trial 3 1.43 1.25 1.22. Wash and scrub the cylinders to clean them.
+/- 0.1 mL (always record to 1 demical places) 100 mL graduated cylinder:. 100mL cylinders have 1ml grading divisions while 10mL cylinders have 0.1 mL grading divisions. There is a line half way around the cylinder to indicate the even number of mL quantities.
The right graduate (example #2)indicates a volume of 4.28 mL (according to the ChemTeam). 50 mL graduated cylinders let you measure volumes up to 50.0 mL to the nearest 0.1 or 0.2 mL, depending on your exact cylinder. We have tutors online 24/7 who can help you get unstuck.
10 ml Graduated cylinder. The copper wire is a great example of an object that would probably be best measured by a more precise ruler. Record the result with the correct number of significant figures in Data Table B.
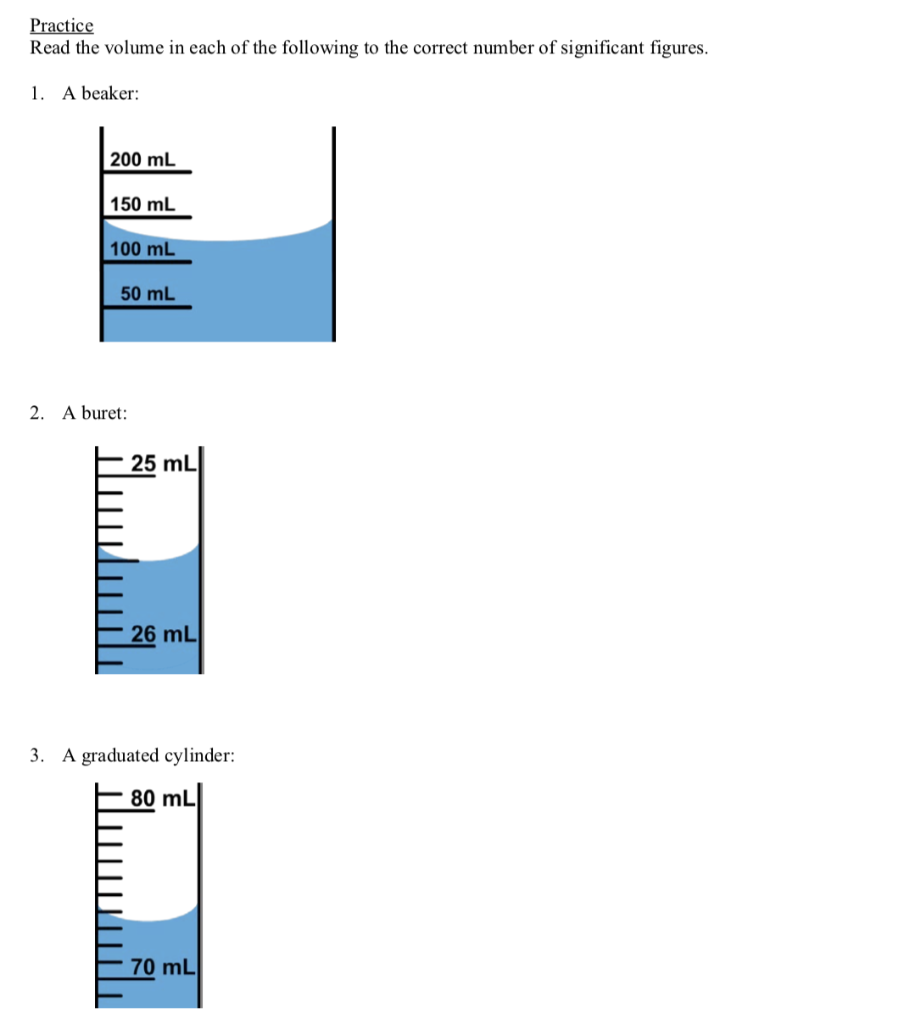
Used a graduated cylinder to measure 25 mL of a solution – What is the difference between the. Calculate the molarity of a potassium dichromate solution prepared by placing 9.67 grams of K 2 Cr 2 O 7 in a 100-mL volumetric flask, dissolving, and diluting to the calibration mark. The bottom of the meniscus in this case clearly lies between the 21 and 22 markings, meaning the liquid volume is certainly greater than 21 mL but less than 22 mL.
The volume of the cube will be measured by volume displacement using a 25-ml graduated cylinder Fill the cylinder with approximately 13 to 15 mL of water. The smallest division is the tenth place, so we are allowed to estimate to the hundredth place. Thus, a 10 mL graduated cylinder will be accurate to within 0.1 mL.
25 mL graduated cylinder This graduated cylinder has white lines going completely around the cylinder to indicate odd number of mL quantities. (25-30 minutes) After students are finished, you should especially review as a class the section with 25 mL graduated cylinders, as these have odd scales that run by 0.2 mL or 0.5 mL. General chemistry scc1.641b afsana abdul rahim lab partner:.
Answer to example #3 Answer to example #4. The level of the water rises to 13.5 mL. Since the smallest division (graduation) is a tenth of a milliliter, we can estimate to a hundredth of a milliliter (0.01).
Flick the cylinders several times to “shake them dry” and dry the outside of the cylinders. 1.7 An object weighing 1.840 kg has a volume of 0.0015 m3. The initial reading of the buret was 9.160 mL and the final was 19.09 mL with 4 significant figures.
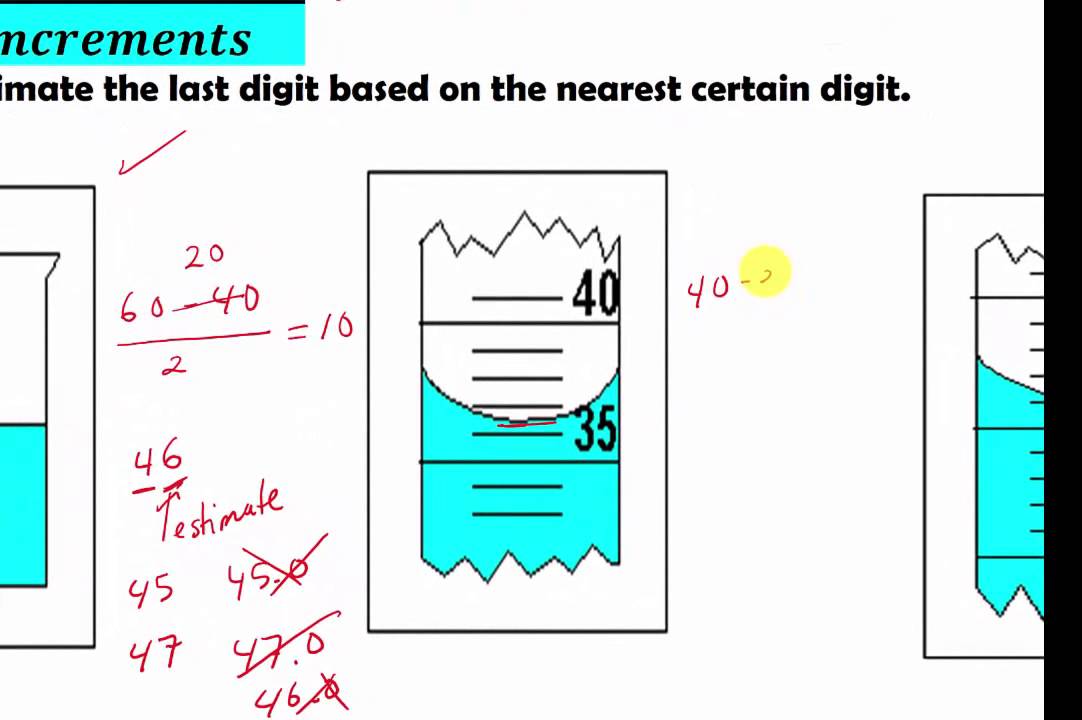
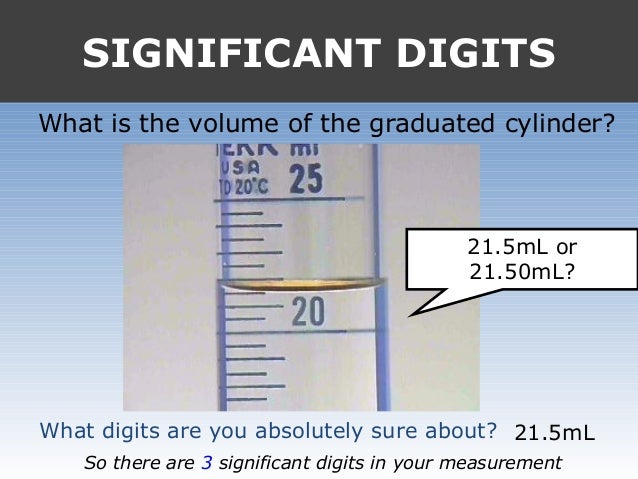
The "2", "0", and "3" we definitely know and the "8" we had to estimate. In the 100-mL graduated cylinder shown, the labeled graduations are 60 and 50 mL. 1.43cm (Based on sig.
V = 26.9 mL V = 35.0 mL Calculations NOTE:. Burets with liquid capacities of 25.00 mL and 10.00 mL are also available. The 25 mL graduated cylinder contained .1 mL with 3 significant figures and the 0 mL graduated cylinder contained the same amount as well.
A 100 mL graduated cylinder is graded in divisions of 1 mL giving results which have 2 significant figures. Below each figure, give the reading for the (i) 50 mL graduated cylinder, (ii) thermometer, an (iii) 25 mL buret with the correct number of…. +/- 0.02 mL (always record to 2 decimal places) 25 mL graduated cylinder:.
I will show an easy way to read a graduated cylinder and a burette with correct significant digits. 421.25 (to four significant figures) 28,6.5 (to five significant figures) Show Answer. What we really mean by this statement is that the meniscus is closer to 42 than it is to either 41 or 43, implying that the actual volume lies somewhere between 41.5 and 42.5 mL.
Volume = mass / density V = 2.25 g / 2.70 g/cm 3 = 0. cm 3. 10 mL graduated cylinders let you measure volumes up to 10.00 mL to the nearest 0.01 mL. They represent a significant improvement in accuracy over beakers and flasks – generally to within 1%.
Significant Figures and the BURET. Assume that 50.mL has two sig figs (it will not. Because of their shape, graduated cylinders fall over easily.
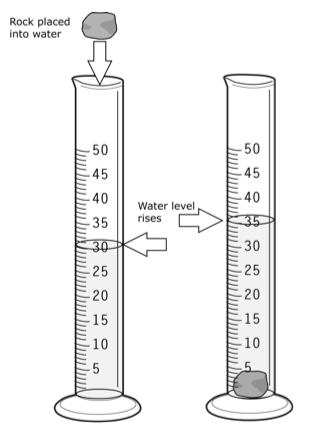
If you look at a 10mL graduated cylinder, for example, the smallest graduation is tenth of a milliliter (0.1mL). Dates Friday, January 13th Lab Recap A - How many digits?. Graduated cylinders are sometimes used to measure the volume of a solid indirectly by measuring the displacement of a liquid.
These vessels can provide precise measurement of liquids or objects, and some styles include a molded-in handle to help provide easy pouring. An equally precise answer would be .39 mL or .37 mL. For more practice on this type of measurement, you can do.
The bottom of the meniscus should be read at eye level. That means when you read the volume, you can estimate to the. A buret is used to deliver a measured amount of liquid into a container.
Weigh the empty cylinders and record the data in the DATA section. Suppose we’re measuring the volume of water in a 50-mL graduated cylinder with markings every 1 mL. This measurement is reported with 3 significant figures and the volume would be 8.68 mL.
Measure out 50 mL of DI water with a 50-mL graduated cylinder. Estimate to the nearest 1/5th mL (1/5th between lines). A sample of metal pellets weighs 13.54 g.
+/- 0.5 mL (always record to 1 decimal place) 500 mL graduated cylinder:. (2 significant figures), otherwise one should use the graduated cylinder (3 significant figures) or better yet, the buret (4 significant figures). Record the volume to 30.1 ml (1 decimal place).
Here are two other graduated cylinder examples:. 27.02 mL, 26.99 mL, 26.97 mL, 27. 25 mL graduated cylinders let you measure volumes up to 25.0 mL to the nearest 0.05 or 0.02 mL, depending on your exact cylinder.
100 ml Graduated cylinder. A 25-mL graduated cylinder has markings every 0.5 mL. How many significant figures does our answer have?.
Read the exact volume at the bottom of the meniscus and record it in Table 1. Be sure to record all significant figures. The graduated cylinder was then placed upright, and the final volume (V) was recorded in units mL:.
• Identify significant figures and use them in mathematical computations. 10 and 25 ml Volumetric pipette. SIG FIGS in reading the GRADUATED CYLINDER.
If the metal is one of the solids listed in Table 1, what is the identity of the metal?. Calculate the mass of the sample. A Graduated Cylinder is not designed to have the highest volumetric accuracy.
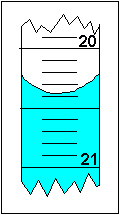
On the right, the liquid level in the 10 mL graduated cylinder is at almost 8.35 mL. Beaker (50-mL) Beaker (1000-mL) Graduated Cylinder (25-mL) Graduated Cylinder (100-mL) Graduated Cylinder (1000-mL) Flask (250-mL) Buret (50-mL) Glass Thermometer Digital Thermometer Top-Loading Balance (400-g) Analytical Balance Uncertainty of Each Device Determine the absolute uncertainty in each device Determine the relative. The density of pure aluminum is 2.70 g/cm 3.
Step 3- Add the cube to the graduated cylinder, being careful not to splash out any water. Aluminum has a density of 2.7 g/mL. 25 to 50 ml Erlenmeyer Flask with solid rubber stopper.
When this sample was placed in a 100-mL graduated cylinder, which contained 25.5 mL water, the new volume (of water and pellets combined) was 27.0 mL. Glass graduated cylinders may chip or break. Glass Graduated Cylinder Set - Thick Lab Cylinders 5ml 10ml 50ml 100ml Measuring Cylinder with 3 Glass Dropper, 2 Brushes and 1 Glass Stirring Rod by FEIGO $15.99 $ 15.
If you again just read to the nearest markings, you’ll get 15.5 or 23.0, etc., i.e., three sig figs. Next, count that there are ten intervals between the labeled graduations. In the graduated cylinder shown in Figure 1, the mL graduations.
Refer to the illustration in Figure 2.1. What is the mass of the aluminum sample?. All calculations must contain the correct number of sig figs!!!.
Therefore, the scale increment is 5 mL/10 graduations = 0.5 mL/graduation. 10 mL graduated cylinder:. For accuracy the volume on graduated cylinders is depicted on scales with 3 significant digits:.
Repeat for a 50 mL graduated cylinder. The buret gave the experimenter 4 significant figures which was the greatest amount of all the glassware. What are the absolute and the relative uncertainties if you deliver 15 mL of a reagent using a 25 mL graduated cylinder?.
Pick up the graduated cylinders you need for your lab at Grainger. You will be using a 25 mL buret with graduations every 0.1 mL. In the 25-mL graduated cylinder, first subtract 25 mL - mL = 5 mL.
2.25 grams of pure aluminum is added to a graduated cylinder containing 11. mL of water. A sample of Aluminum is placed in a 25 mL graduated cylinder containing 10.0mL of water. Most liquids, such as water, form a concave meniscus (see Figure 1).
What is the density (g/mL) of an object that has a mass of 14.01 grams and, when placed into a graduated cylinder, causes the water level to rise from 25.2 mL to 33.6 mL?. Graduated glassware is used to measure liquid volumes. We examine the meniscus and decide it is closest to 42 mL.
If an instrument does have markings that are powers of ten, then finding the precision of the instrument is easy. 1.7 An aluminum engine block has a volume of 4.77 L and a mass of 12. kg.

Measurement And Significant Figures Youtube

Graduated Cylinder Wikiwand

Significant Figures Chemclass Ol Org

Reading A Graduated Cylinder Worksheet Printable Worksheets And Activities For Teachers Parents Tutors And Homeschool Families
Web Gccaz Edu Rob Safety density Density Bkgd Pdf

Significant Figures Pathways To Chemistry

Thick Glass Graduated Measuring Cylinder Set 5ml 10ml 50ml 100ml Glass With Two Brushes Amazon Com Industrial Scientific

Metric Measurement Lab Objective Materials Procedure Accuracy And Precision Significant Figures
Pdf Document Capuchem Ca Capu Logo

Lab 1
Www Hasdk12 Org Site Handlers Filedownload Ashx Moduleinstanceid Dataid Filename Lab introduction to measurement Pdf

Lab 1

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry

Lab Notes Significant Figures Narrated Authorstream

Uncertainty In Lab Measurements And Reading Volumes Purpose

Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry

I Need Help With This Question A Piece Of Zinc Copper Alloy Weighs 25 G It Was Placed Into A Graduated Cylinder And The Volume Of The Water Is Increased By 3 2 Ml

Experiment 1 Density And Measurement Chem 1100 Studocu

Cp03 Sig Figs Chemteacher

Starter The Radius Of The Moon Is 1 737 000 Meters Write This In Scientific Notation The Diameter Of A Carbon Atom Is Meters Write Ppt Download

Read Measurement Of Graduated Cylinder Beaker And Flask Youtube
2 1 Significant Figures Introductory Chemistry
Www Lacitycollege Edu Departments Chemistry Documents Chemistry 60 Experiments Documents Introduction To Measurements
How To Read A Graduated Cylinder Or A Burette Youtube
Chemistry Lab 1 Measurements Chem 1211 Studocu
Experiment 1 Basic Measurement Techniques

Ppt Scientific Measurement Powerpoint Presentation Free Download Id
.PNG)
Scientific Measurement Presentation Chemistry

Introduction Estimating And Uncertainty Accuracy And
Significant Figures

Level 4 Significant Figures Reading A Graduated Cylinder Using Correct Significant Figures Diagram Quizlet

2 4 Significant Figures Chemistry Libretexts

Thick Glass Graduated Measuring Cylinder Set 5ml 10ml 50ml 100ml Glass With Two Brushes Amazon Com Industrial Scientific

Data Laboratory 2 Part 1 1 Temperature Of The Di Chegg Com
2
Q Tbn 3aand9gcr18zsswwci6alk7xewmhsmkvx3lm Fnag24m Yggy Usqp Cau

Metric Measurement Lab Objective Materials Procedure Accuracy And Precision Significant Figures
Ch 2 Data Analysis
Question 945e2 Socratic

Sig Figs Grad Cyl

Solved T Laboratory Questions You Performed A Calculatio Chegg Com

2 4 Significant Figures Chemistry Libretexts

Math Skills Scientific Notation

Significant Figures Chemclass Ol Org

25 Ml Graduated Cylinder Glass Measuring Cylinders 25cc Pyrex Kimax
2
Myweb Ntut Edu Tw Chpro Chem Chap1 Pdf

Significant Figures Lab Middlebury College Chem 103 Lab
Significant Figures

Laboratory Techniques And Measurements

1 5 Measurement Uncertainty Accuracy And Precision Chemistry
Http Mrsq Net Images Density And Percent Error Notes Pdf
01 Significant Digits
.PNG)
Scientific Measurement Presentation Chemistry

Accuracy And Precision Of Laboratory Glassware
Http Dpuadweb Depauw Edu Harvey Web Etextproject Ac2 1files Chapter2 Pdf
Significant Figures

Sig Figs Grad Cyl
Www Michigan Gov Documents Mdch Measures Power Point 7 Pdf

Graduated Cylinder Wikipedia

Lab 1 Ivy Tech Chemistry 101

Ppt Measurement Powerpoint Presentation Free Download Id
Q Tbn 3aand9gcs Vdjjob77ngotuwmk317k5g8nko9gqxzz6t Zvxk Usqp Cau
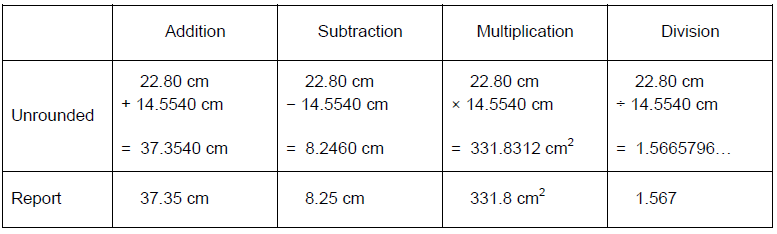
Solved Practice Read The Volume In Each Of The Following Chegg Com

1 9 Essential Skills 1 General Chemistry
Reading The Volume From A 25 Ml Graduated Cylinder
How Would You Determine The Proper Number Of Significant Figures For Measurement Of A Liquid Using A Graduated Cylinder Quora
Math Skills Scientific Notation

Properties Of And Changes In Matter Ppt Download
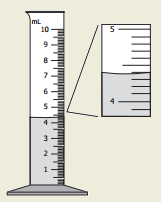
High School Chemistry 09 Released Test

Significant Figures Ppt Download

Graduated Cylinder Worksheets Teaching Resources Tpt
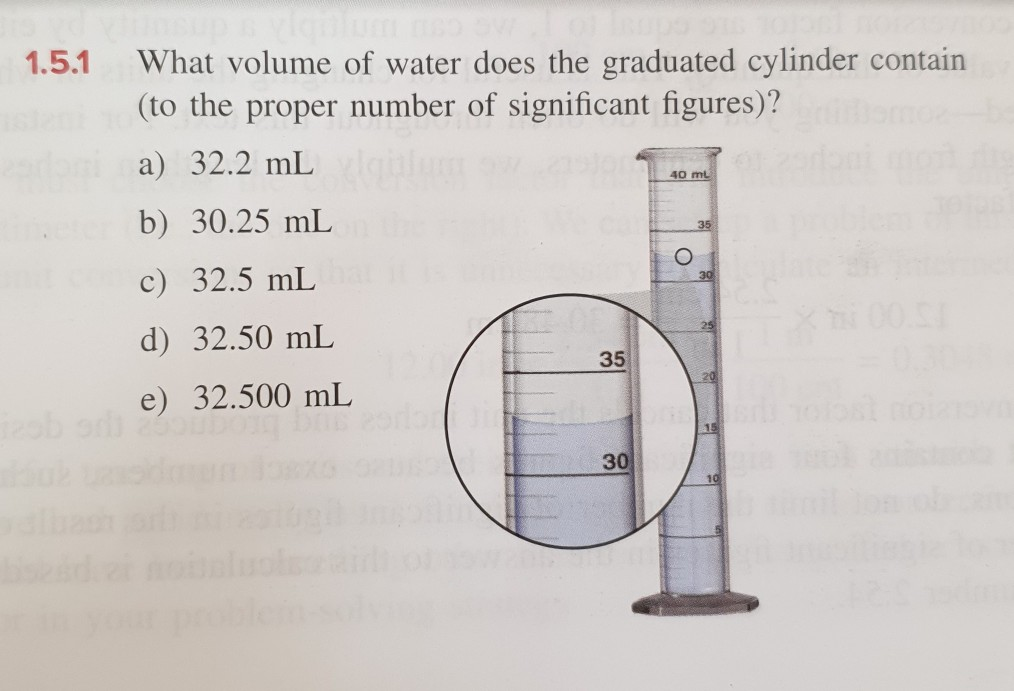
Solved Answer C But Why D And E Are Wrong Chegg Com
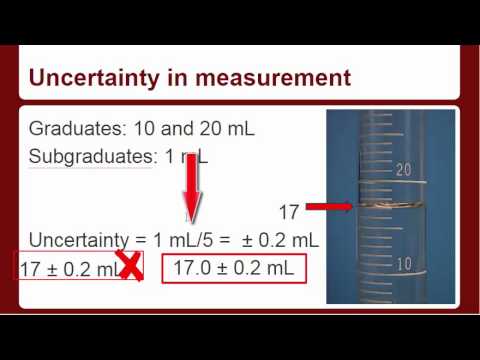
Reading Graduated Cylinders And Uncertainty Youtube
2
Www Michigan Gov Documents Mdch Measures Power Point 7 Pdf
Q Tbn 3aand9gcrq9ii Jj9gidjsp9be0ptxqo0f14tf15z0odto2nnpjooigsxe Usqp Cau
.PNG)
Scientific Measurement Presentation Chemistry
3

Graduated Cylinder Wikipedia

Lab 1

What Is The Volume Of The Liquid In The Graduated Cylinders Answer To The Correct Number Of Significant Figures Study Com
Http Faculty Sites Uci Edu Chem1l Files 13 11 Rdgvolflaskpipet Pdf

Chm 101 102 Laboratory Manual Significant Figures And Density General Chemistry 101 102 Laboratory Manual University Of North Carolina At Wilmington Ppt Download

Graduated Cylinder Wikipedia

Using Sigfigs When Measuring With An Instrument With Marks Other Than Powers Of Ten Chemistry Stack Exchange
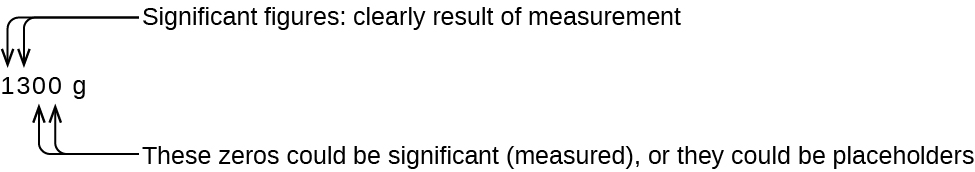
1 5 Measurement Uncertainty Accuracy And Precision Chemistry

Bill A Lab Technician Was Asked To Measure Out Sodium Hydroxide Naoh For An Experiment In A Brainly Com
Zumdahl Chapter 1 Uncertainty In Measurement

If You Measure Water Of A Graduated Cylinder And Find It To Be 23 Would You Write It As 23 Ml Or 23 0 Ml Quora

Significant Figures Chemclass Ol Org
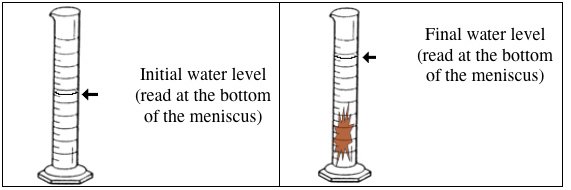
Initially 10 0 Ml Of Water Was Placed In A Graduate Cylinder And Then A Solid Metal Was Immersed In The Water In The Graduated Cylinder At This Point The Volume Of Water
2

Precision Of Lab Equipment Question Chemhelp
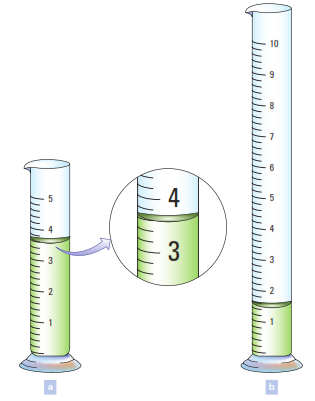
State The Volume Of Liquid In Each Graduated Cylinder In The Figure Below And Explain How You Decided Upon The Appropriate Number Of Significant Figures The Markings Are Calibrated To Be Read
Laboratory Techniques And Measurements

Chemistry Tutorial 2 Flashcards Quizlet

Significant Figures Chemistry Video Clutch Prep



