Pcl3 Lewis Structure With Charges
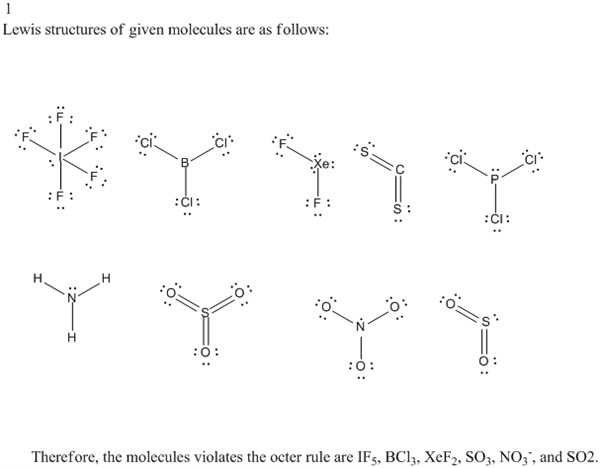
Solved 1 Look Again At The Lewis Structures That You Dre Chegg Com

Ppt Video Online Download
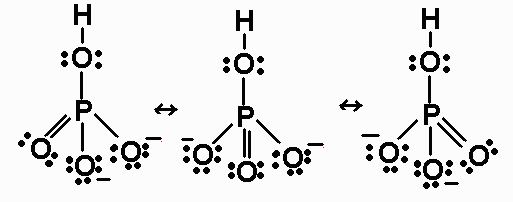
Untitled

Oneclass Consider The Lewis Structure For Pcl3 What Are The Appropriate Bond Angles
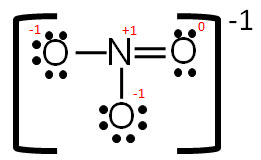
Covalent Bonding Electron Dot Diagrams Texas Gateway
Q Tbn 3aand9gcqrbeh4djrpb8 Ish 18oj5uz30k3kd5gdpcajena2uu11f7 X Usqp Cau
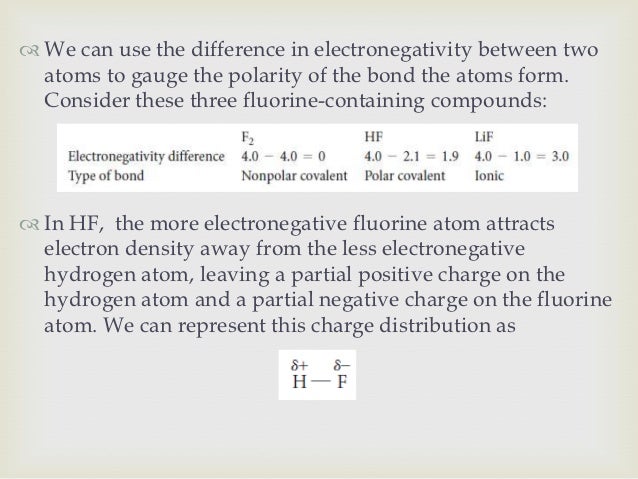
It is the well-known fact that if there is a vast difference of the electronegativity, there are more chances of polarity.

Pcl3 lewis structure with charges. N = 5 – 4 - ½(4) = -1;. A step-by-step explanation of how to draw the POCl3 Lewis Structure. Thus, the Lewis structure of NO is.
It is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds for a wide variety of applications. Dipoles & Dipole Moments:. CO3, OH-, NO3 etc), so how is it polyatomic compound when it looks like an ionic compound?.
1, resulting in a polar covalent bond. In a Hoechst continuous process, molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. The “best” Lewis structure is one that has the fewest formal charges — the top structure.
(a) the amino acid serine:. This results in a bent structure that thereby unequally distributes charge throughout the molecule inducing a permanent dipole. Tell me about the best Lewis structure.
Draw the Lewis structure for the molecule or ion. Try to draw the PCl 5 Lewis structure before watching the video. Tell me about the best Lewis structure.
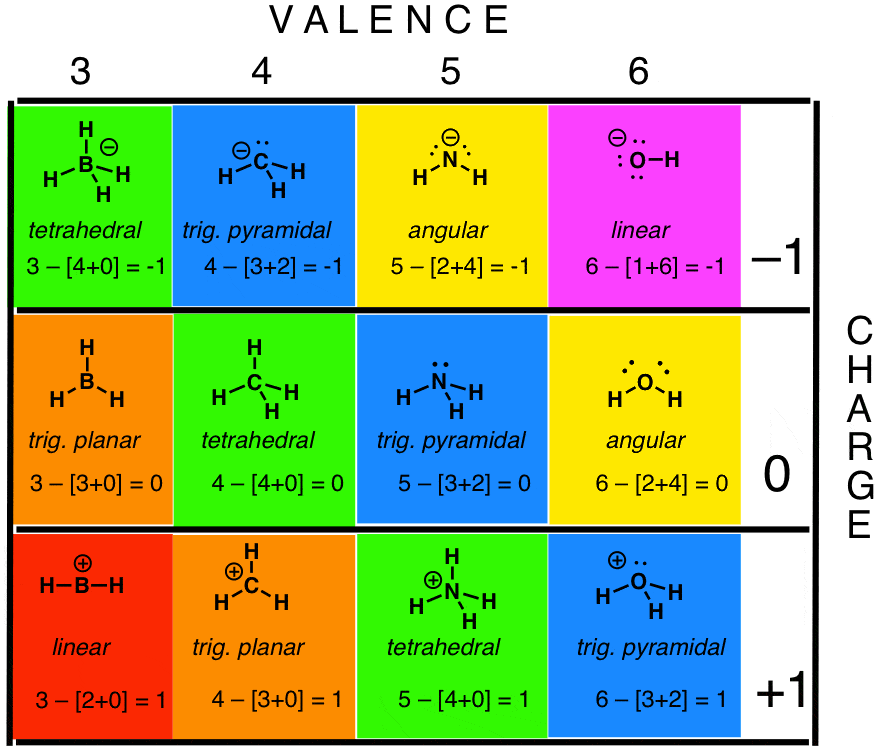
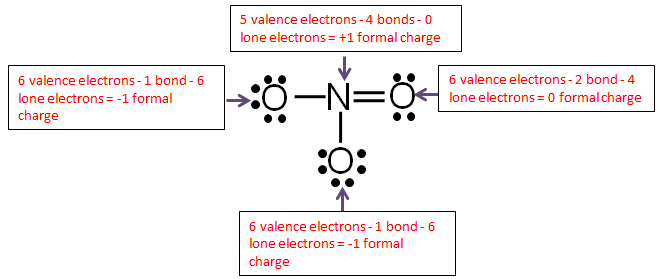
Answer All Questions Related To The Drawing Underneath Draw The Lewis Structure What Is The Geometric Shape For The Central Atom What Is The Formal Charge What Is The Hybrid Orbital Designation For The Central Atom How Many Sigma Bonds In The Formula How Many Pi Bonds In The Formula Draw The Lewis Structure For ClNO. The Lewis structure of ozone (O 3) 1. The formal charge of an atom in a Lewis structure is the charge the atom would have if all bonding electrons were shared _____ between the atoms.
Lewis structures, also known as electron dot structures, are named after Gilbert N. (Assign lone pairs, radical electrons, and atomic charges where appropriate.) ER:. We look at formal charge (Google this one) and find out that the charge is 0 on all atoms in this compound.
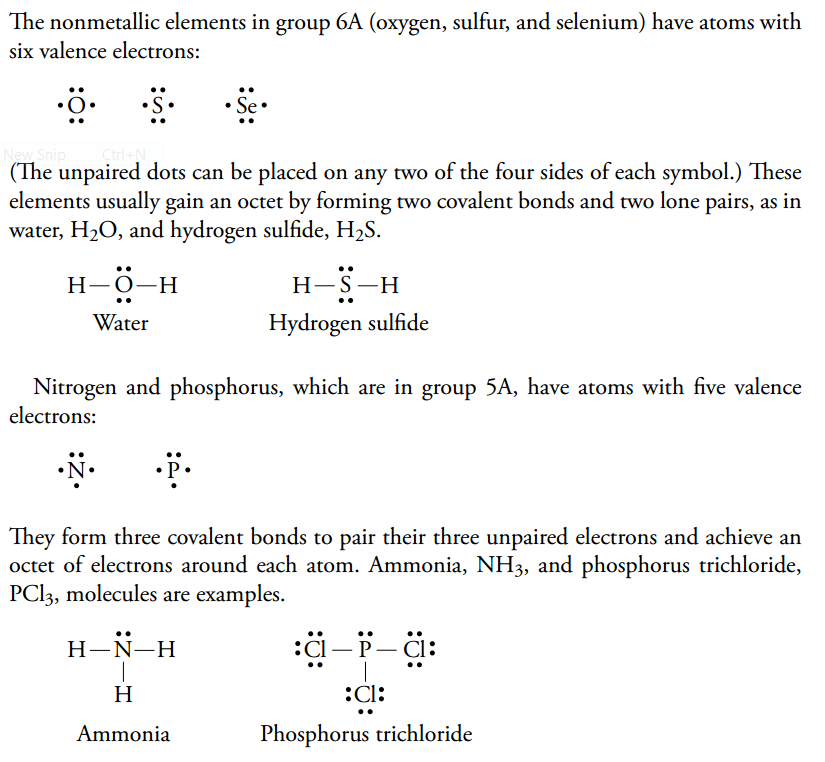
Writing Lewis Structures PCl3 Find the sum of valence electrons of all atoms in the polyatomic ion or molecule. Lewis Structures Lewis structures are representations of molecules showing all electrons, bonding and nonbonding. We divide the bonding electron pairs equally for all I–Cl bonds:.
Charges and Resonance Page 4 of 8 62. Atomic Charges and Dipole Moment P1 charge= 0.286 CL2 charge= 0.002 O3 charge=-0.294 CL4 charge= 0.002 CL5 charge= 0.002 with a dipole moment of 2.4 Debye Bond Lengths:. The formal charge does not necessarily reflect an actual charge on the atom.
The Lewis structure of SO2Cl2 is constructed as follows:. Apr , 18 - A step-by-step explanation of how to draw the PCl3 Lewis Structure (Phosphorus Trichloride). Single bond the two Cl atoms 180 degrees away from each other to the S atom.
Electrons Required to give all atoms except hydrogen an octet and hydrogen a duet VE:. For the Lewis structure for PCl5 you should take formal charges into account to find the best Lewis structure for the molecule. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule,.
Place the S atom at the center. Single, Double & Triple Bonds. If it is an anion, add one electron for each negative charge.
Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. In the POCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. I'm told that PCl5 is Polyatomic compound HOWEVER neither element is listed in my list of polyatomic ions (ex:.
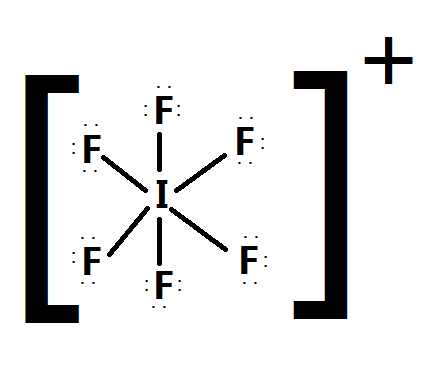
Calculating Formal Charge from Lewis Structures Assign formal charges to each atom in the interhalogen ion ICl 4 −. 0, 0 (7 - 1/2 (2)-6= 0) both have 7 valance e-How many covalent bonds are in the Lewis Structure of CH3CHCl2?. The way he told us to set up the Lewis Structure is H O Br O but every way I add bonds and electrons, I'm not getting the answer for the formal charges, which is the O farthest to the right as -1 and the Br as +1.
N = 5 - 3 - ½(4) = 0;. Each Cl atom now has seven electrons assigned to it, and the I atom has eight. Lewis dot structure for the \(NO^+\) ion with ten valence.
CN – Answer a. Solution The structure with formal charges of 0 is the most stable and would therefore be the correct arrangement of atoms. In the Lewis structure for POCl 3 there are a total of 32 valence electrons.
Nitrogen monoxide has 11 valence electrons (Figure 1). NO – N 2;. The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorus.The heat of reaction, ca.
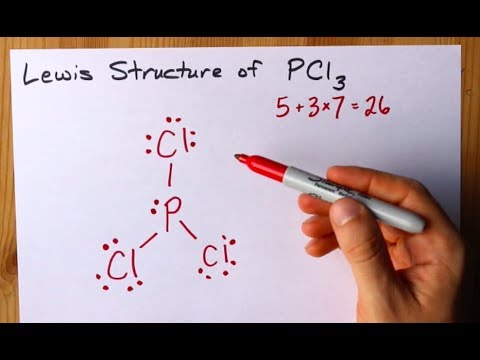
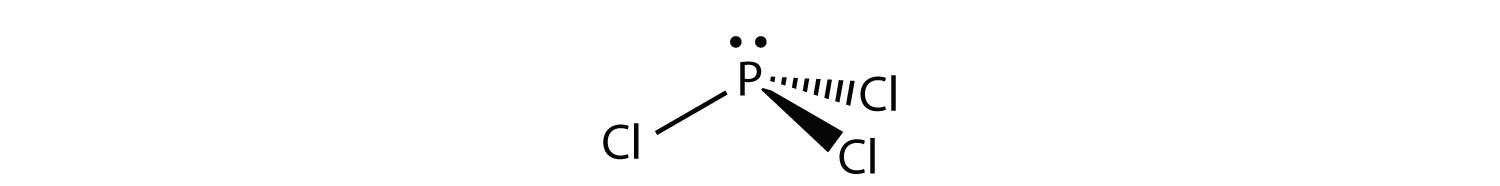
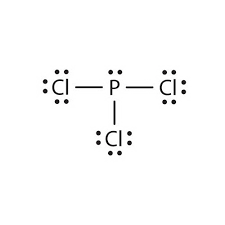
Valence electrons available to all the atoms. The structure of PCl3 is a phosphorus with a lone pair (two electrons) and 3 chlorine atoms attached by a single bond where each chlorine has 3 lone pairs. #N_7rarr1s^(2)2s^(2)2p^(3)# Hence, the nitrogen atom is devoid of any sort of #d# orbital.
This is a reasonable Lewis structure, because the formal charge on all atoms is zero, and each atom (except H) has an octet of electrons. It is helpful if you:. Lewis structures that minimize formal charges tend to be lowest in energy, making the Lewis structure with two S=O double bonds the most probable.
It is a toxic and volatile liquid which reacts violently with water to release HCl gas. The phosphorus has an electronegativity value of 2.19 and chlorine comes with 3.16. Tell me about the atomic charges, dipole moment, bond lengths, angles, bond orders, molecular orbital energies, or total energy.
Lewis Structures, Formal Charge, and Geometry Worksheet 7 1. Several worked examples relevant to this procedure were given in previous posts please see the Sitemap - Table of Contents (Lewis Electron Dot Structures). The following molecules have only single covalent bonds.
Determine the formal charge of each element in the following:. Lewis dot structure for a PCl3 molecule:. Calculate the formal charge of chlorine in the molecules Cl 2, BeCl 2, and ClF 5.
<p>The steric number of 'P' is 5. State the hybridization of the central atom in the stru. On the periodic table phosphorus charge is 3- and chloride charge is -1.
Calculating the formal charge for a molecule allows you to determine which resonance structure is more likely to be the molecule’s correct structure, and the Lewis structure which is considered the most correct will be the structure that has. Now if we look at the electronic structure in the ground state of phosphorus it will be 1s 2, 2s 2, 2p 6, 3s 2, 3p 2.The valence shell in phosphorus is 5. A simple procedure for writing Lewis dot structures was given in a previous post entitled “Lewis Structures and the Octet Rule”.
Phosphorus trichloride is a chemical compound of phosphorus and chlorine, having the chemical formula PCl3. When the following become IONS what will the charge be?. Use the Lewis structure to predict the molecular geometry.
Thus far, we have used two-dimensional Lewis structures to represent molecules. Determine the formal charge of each element in the following:. I'm confused where the ionic charge of 5 came from.
Lewis structures, also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. The formal charge on each atom is:. Molecule Polarity </p> <p> The hybridization.
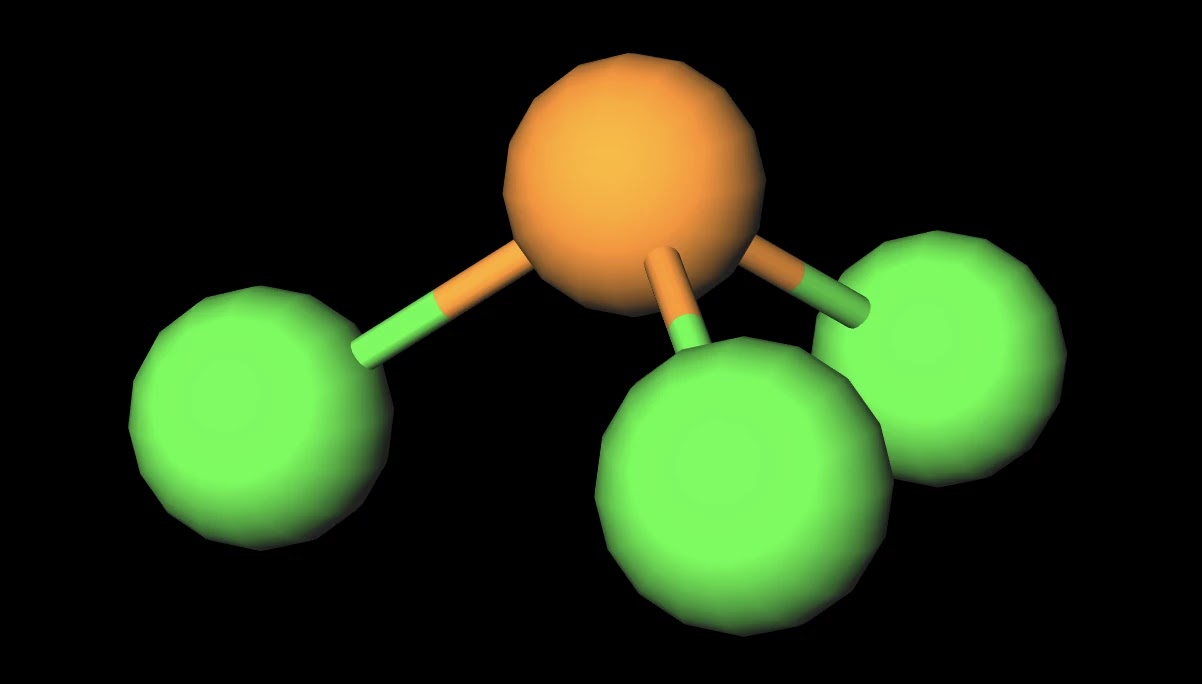
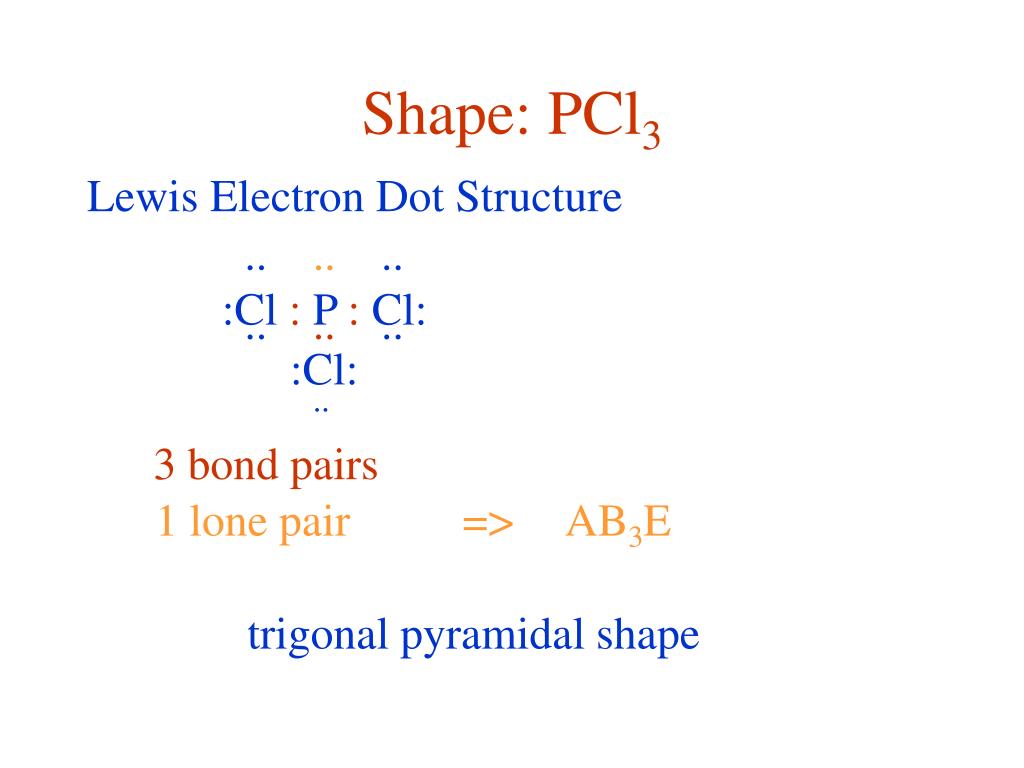
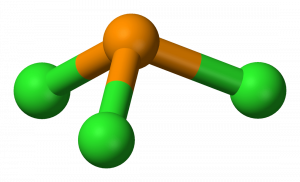
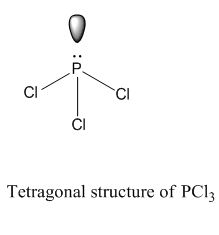
Resonance structures are all of the possible different Lewis structures that a molecule might have. It is trigonal pyramidal in shape. Hree pairs will be used in the chemical bonds between the P and Cl.
Do not add any more atoms. 2.4K views · View 2 Upvoters. We can write two possible structures.
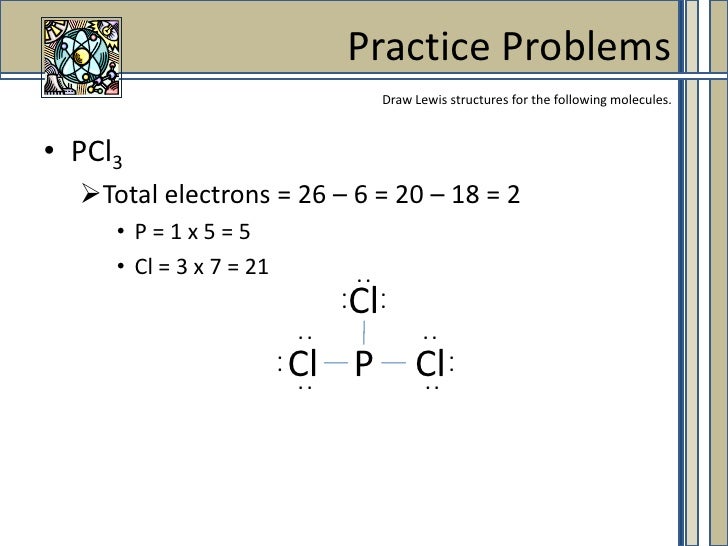
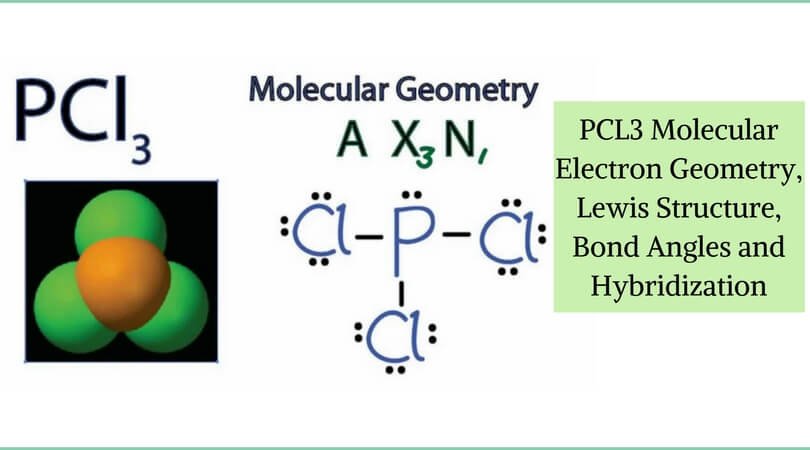
PCl3, phosphorus is in the middle with one lone pair and is surrounded by single bonds to the three chlorines, each with 3 lone pairs. For the PCl3 Lewis structure we first count the valence electron. In the Lewis structure for PCl 3 there are a total of 26 valence electrons.
Write Lewis structures for the following:. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule." Lewis structures depict the bonds between atoms of a molecule, as well as any unbonded electron pairs. O = 6 – 4 - ½(4) = 0 Bottom structure:.
It has a trigonal pyramidal shape, owing to the lone pairs on the phosphorus. If the Lewis structure must have nonzero formal charges, the arrangement with the smallest nonzero formal charges is preferable. Lewis structure of HBrO2 and formal charges?.
Draw a Lewis structure for each. Draw Lewis structures for each. Using Formal Charge to Predict Molecular Structure.
If you need more information about formal charges, see Lewis Structures. Write the Lewis structure for a molecule of. Draw The Lewis Structure Of PCl3.
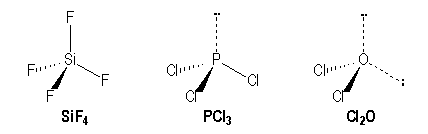
Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign. If we look at the molecule of PCl3 it is made up of phosphorus and chlorine molecules However, the hybridization basically occurs within the central atom which is phosphorus. Congrats, you are done.
We assign lone pairs of electrons to their atoms. (a) HCl (b) CF 4 (c) PCl 3 (d). In the Lewis structure of ClF, the formal charge on Cl is _____, and on F is _____.
In the PCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. Draw the Lewis structures and the resonance structures for NO3 –. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
See page 637 of your text. O = 6 - 3 + ½(4) = +1. The electronic configuration of nitrogen #(N)# is:.
K+is normally drawn with zero dots because K just lost its only valence electron (although K+of course has 8 valence electrons just like argon). A step-by-step explanation of how to draw the PCl3 Lewis Structure (Phosphorus Trichloride). For the PCl3 Lewis structure we first count the valence electron.
SCl2, OCl2, PCl3, SiCl4, SiCl62−. When we know the number of electrons, we can then build the Lewis dot structure in the usual manner. PCl3 is a polar molecule due to the presence of a lone pair of electrons at the top of the molecule leading to electron-electron repulsion.
The arrangement of atoms in a molecule or ion is called its molecular structure.In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure—different multiple bond and lone-pair electron placements or different arrangements of atoms, for instance. PCl 3 is not trigonal planar. If we were to consider the nitrogen monoxide cation (\(NO^+\) with ten valence electrons, then the following Lewis structure would be constructed:.
Atomic Charges and Dipole Moment P1 charge= 0.229 CL2 charge=-0.076 CL3 charge=-0.077 CL4 charge=-0.076 with a dipole moment of 0. Debye Bond Lengths:. For each of the formulas in the table below draw the Lewis structure and enter the number of the following. H 2 CCH 2;.
For example, for NO3 –, the formal charge on the nitrogen and oxygen atoms must add up to –1. A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. For ionic compounds, the formal charges on the atoms must add up to the charge on the ion.
Lewis Structure (+VSEPR) for H2CO - Duration:. The following structures may have a double, triple, or coordinate covalent bond. So all the Chlorines have octets.
7 (how many non-metals & metals are bonding) How many equivalent resonance forms can be drawn for CO32−?. The negative charge generally resides on the more. All of the Chlorines, they're symmetrical, and they have 2, 4, 6, plus this line right here, 8 valence electrons;.
The molecular geometry of PCL3 is trigonal pyramidal with the partial charge distribution on the phosphorus. Write Lewis structures for the hydrogen carbonate ion and hydrogen peroxide molecule, with resonance forms where appropriate. It has a 31P NMR signal at around +2 ppm with reference to a phosp.
10 times the heat of evaporation, keeps the system at its boiling point, and the phosphorus trichloride distills off. Draw the Lewis structure of the NO^-_2 ion and clearly show your working. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space ().A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.
Double bond the S atom to 2 O atoms 180 degrees from each other. Tell me about the atomic charges, dipole moment, bond lengths, angles, bond orders, molecular orbital energies, or total energy. What is the Hybridization of Phosphorus Trichloride?.
A molecular structure in which all formal charges are zero is preferable to one in which some formal charges are not zero. The total number of ten comes from the carbon atom (four), the nitrogen atom (five), and the -1 charge (1). Step 2 Lewis structures of given molecules are given below,.

Which One Of The Following Molecules Has Two Lone Pairs Of E Clutch Prep

Chapter 8 Basic Concepts Of Chemical Bonding Ppt Download

Chapter 10 Flashcards Quizlet

Model Systems For A Stepwise Hydrolysis Of A Pcl3 B P Oh Cl2 C Download Scientific Diagram

Solved 9 Draw The Lewis Dot Structure For The Following Chegg Com

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Chemed Dl Application Models 360
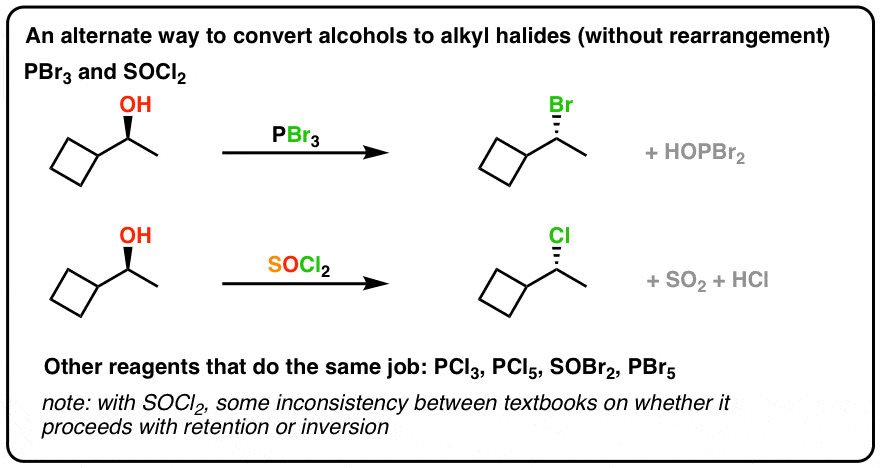
Pbr3 And Socl2 Master Organic Chemistry

Phosphorous Trichloride Pcl3 Lewis Dot Structure Youtube

Problem Set 6

Formal Charges And Resonance Chemistry For Majors

Is Pcl3 Polar Or Nonpolar Study Com

Oneclass Write Lewis Structures For Pcl3

1 Electronegative Electropositive Concepts Pages 1 50 Flip Pdf Download Fliphtml5
How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube

Chem Completing The Octet Scientific Tutor
Vsepr Method By G Dupuis And N Berland
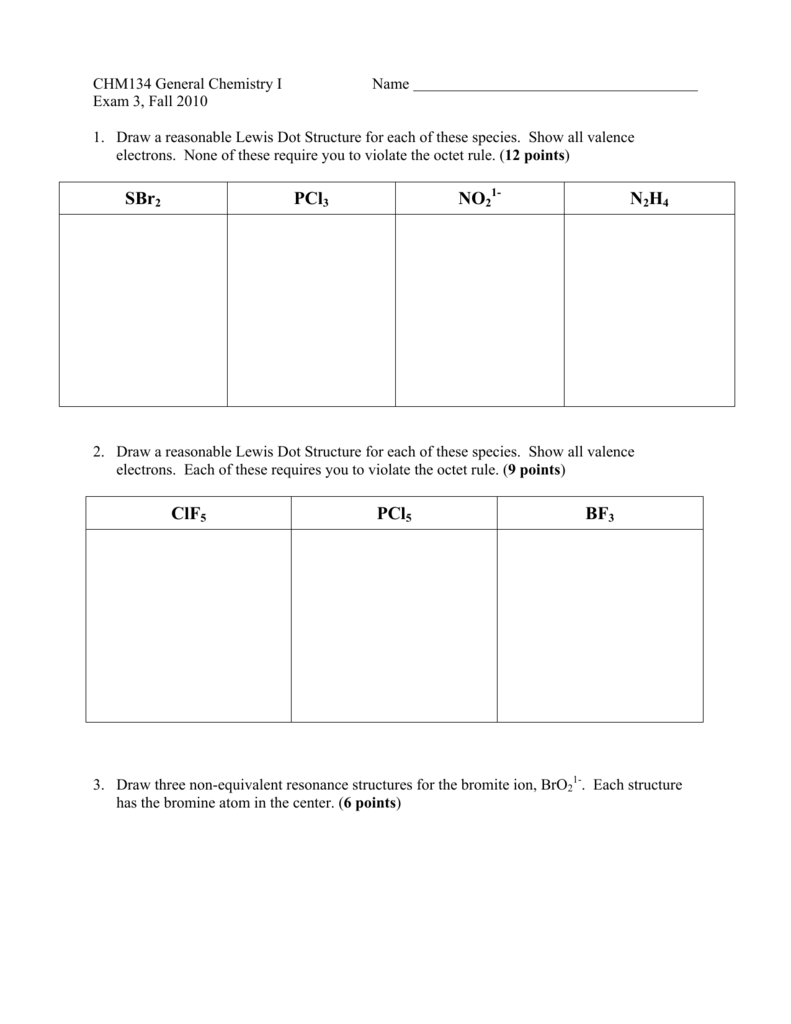
Sbr2 Pcl3 No2 N2h4 Clf5 Pcl5 Bf3
Chemical Bonding Sk0023
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library
7 3 Lewis Symbols And Structures Chemistry

10 Lewis Structures Chemistry Libretexts
Q Tbn 3aand9gcsnqi 50afiqze8rddi6wm5w Iktp9vd4y0dis4gwqdajeik3m0 Usqp Cau
Is Pcl3 Polar Or Nonpolar
Solved 1 Complete The Following Table For 5 Molecules D Chegg Com

Answers Explaining Shapes

Lewis Dot Structure Of Pcl3 Phosphorous Trichloride Youtube

Lewis Structures Ac
How To Calculate Formal Charge

Why Is Bf3 Written In Two Ways Quora

Model Systems For A Stepwise Hydrolysis Of A Pcl3 B P Oh Cl2 C Download Scientific Diagram

Determine The Formal Charge On The Iodine Atom In The Hio Molecule Study Com
Fresininda
Solved Draw The Lewis Structure For Nobr In The Window Be Chegg Com
1

Test Bank For General Chemistry 10th Edition By Ebbing By Zoqqa139 Issuu
Examview Ch 9 10 Practice Test Tst

Lewis Structures And Hybridizations For Common Molecules
Ppt Molecular Geometry And Chemical Bonding Theory Powerpoint Presentation Id

Provide The Lewis Dot Structure And All Resonance For The Ion Rncl2 2 Sbcl5 Pcl3

What Type Of Bond Is Pcl3

Covalent Bonding Lewis Structures Ppt Video Online Download

Pcl5 Lewis Structure

Reaction Of Alcohols With Pcl5 And Pcl3 Chemistry Stack Exchange
Q Tbn 3aand9gcqd4mttlm Lxof4eicktwu61s1r1uqgmy1pgwcrao8cnbdkit O Usqp Cau

Bonding And Shape Hl

Simple Bonding Theory Review Of Lewis Structures And Vsepr Theory Slideshow And Powerpoint Viewer Resonance Some Molecules May Have More Than One Valid Lewis Structure These Structures Differ In

Is Pcl3 Non Polar Or Polar Why Quora
Covalent Bonding Electron Dot Diagrams Texas Gateway
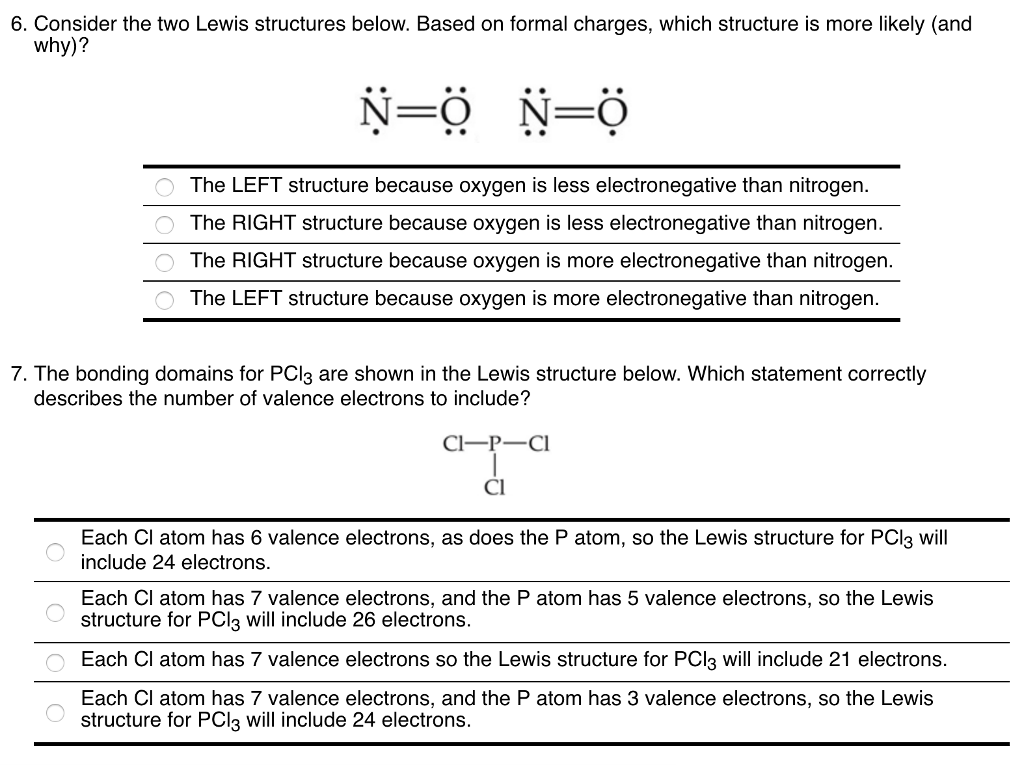
Solved 6 Consider The Two Lewis Structures Below Based Chegg Com

Diagram Nf3 Lewis Diagram Full Version Hd Quality Lewis Diagram Orquestralivre Arcieriarcobaleno It
Phosphorus Trichloride Pcl3 Pubchem
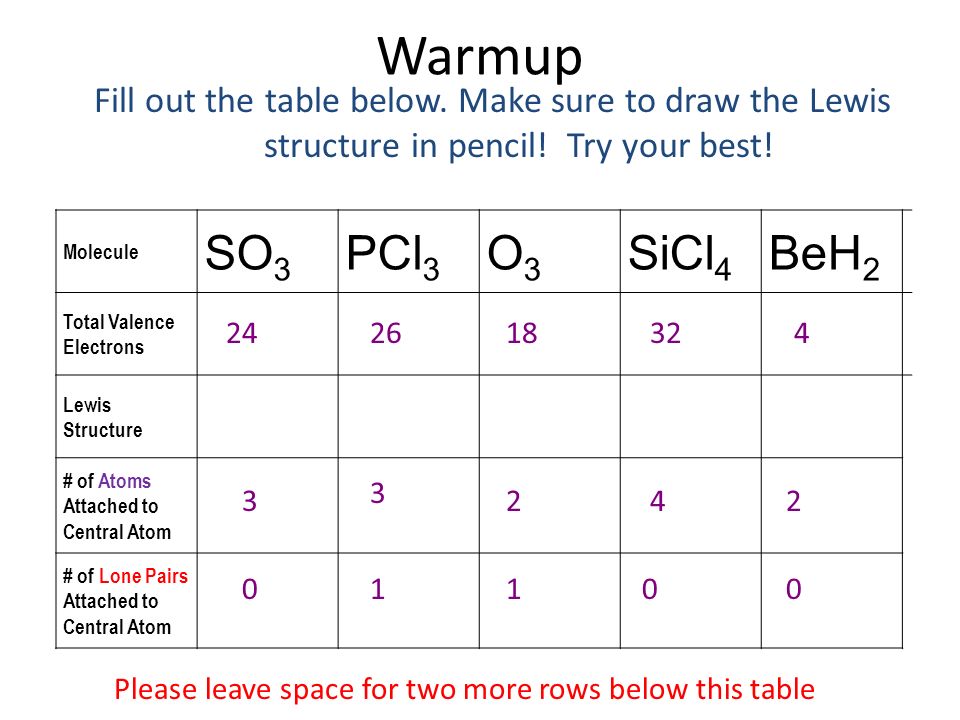
Warmup Fill Out The Table Below Make Sure To Draw The Lewis Structure In Pencil Try Your Best Molecule So3 Pcl3 O3 Sicl4 Beh2 Total Valence Electrons Ppt Video Online Download

How Is The Electron Dot Structure Of Pcl3 Determined Quora

Which Of These Molecules Are Polar Check All That Apply Pcl3 So2 Co2 Ch2cl2 Homeworklib

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Molecular Geometry Shape And Bond Angles Youtube
Cnx Chemistry Ssm Ch07 Mod04 Sci105 Usc Studocu
Lewis Structures Ac
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Chapter 10 Flashcards Quizlet
Dot Diagrams Kaiserscience

Determine The Formal Charge Of Each Elemen Clutch Prep
Piazza Com Class Profile Get Resource Iog601eydm5tk Is0shpzcbbg636

Occurrence Preparation And Properties Of Phosphorus Chemistry Openstax Cnx

Chemistry 114 Chapter 10 Flashcards Quizlet

Formal Charges And Resonance Chemistry For Majors

Exceptions To The Octet Rule Chemistry Master
Pcl3 Lewis Structure

Pdf First Principles Computational Study On Hydrolysis Of Hazardous Chemicals Phosphorus Trichloride And Oxychloride Pcl 3 And Pocl 3 Catalyzed By Molecular Water Clusters
Sf4 Molecular Geometry Lewis Structure And Polarity Explained
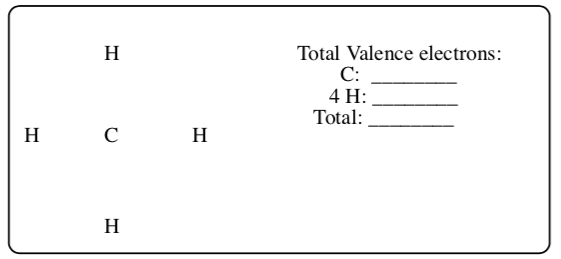
10 Lewis Structures Chemistry Libretexts

Chapter 8 Concepts Of Chemical Bonding 8 5 To 8 8 Ppt Download
Www Mctcteach Org Chemistry C10 C10 Handouts Molecular modeling v 8 18 Pdf

Chapter 6 The Shape Of Molecules Ppt Download
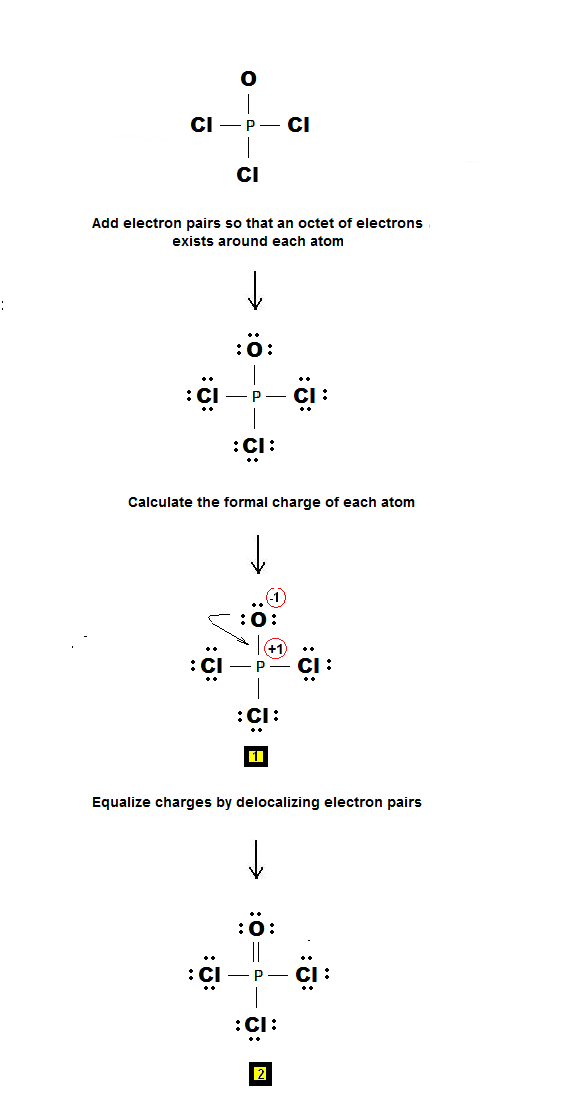
Chemistry Net How To Draw Lewis Dot Structures Pocl3 Phosphorous Oxychloride

Chap01 Intro 0 Docx Ligand Coordination Complex

Molecular Geometry Post Lab 3 1 35 36points Post 001 Assignlonepairs Radicalelectrons Andatomic Er El Course Hero

Ppt Chm 45 Molecular Geometry Chemical Bonding Chapter 10 Powerpoint Presentation Id
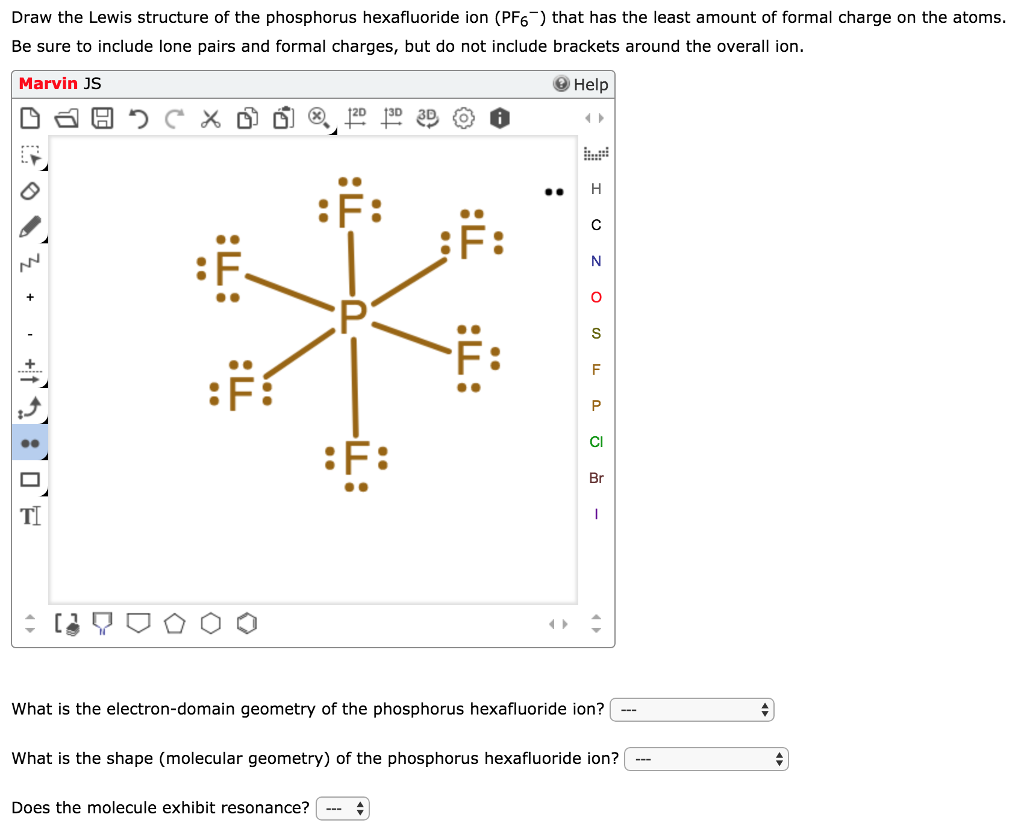
Diagram Lewis Dot Diagram Of Phosphorus Full Version Hd Quality Of Phosphorus Silverfusa110 Fujiya It

Rank The Members Of The Set Of Compounds Pcl3 Pbr3 Pf3 In Order Of Decreasing Ionic Character Of Their Bonds Use Par Homeworklib

Lewis Symbols And Structures Introductory Chemistry

What Type Of Bond Does Pcl3 Have Quora

Phosphorous Bonding In Pcl3 H2o Adducts A Matrix Isolation Infrared And Ab Initio Computational Studies Sciencedirect

Pcl3 Lewis Structure How To Draw The Lewis Structure For Pcl3 Phosphorus Trichloride Youtube

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Chapter 2 Questions
Bond06

Phosphorus Pentachloride Wikipedia
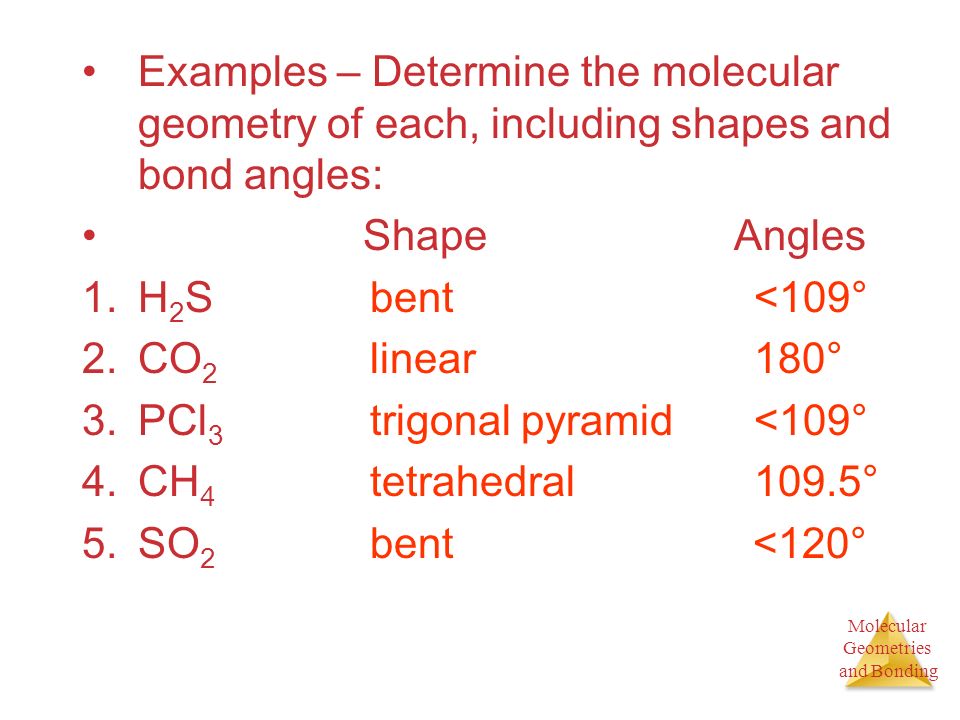
What Type Of Bond Is Pcl3

Pcl3 Lewis Structure How To Draw The Lewis Structure For Pcl3 Phosphorus Trichloride Youtube

Determine The Formal Charge Of Each Elemen Clutch Prep
What Type Of Bond Is Pcl3
Http Sites Uci Edu Chemcommonfinal Files 16 03 Midtermii Example Pdf
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization



