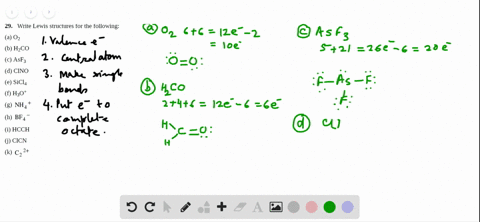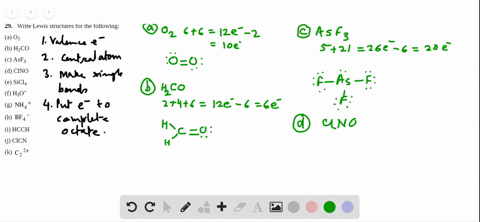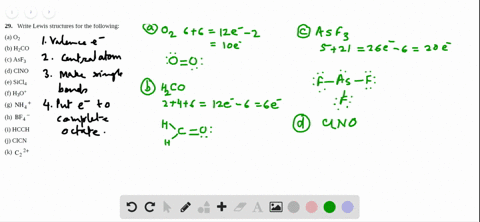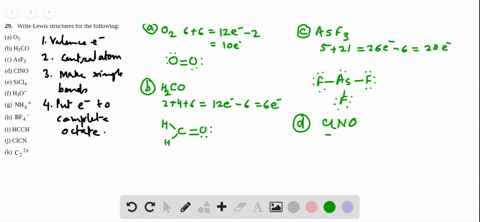
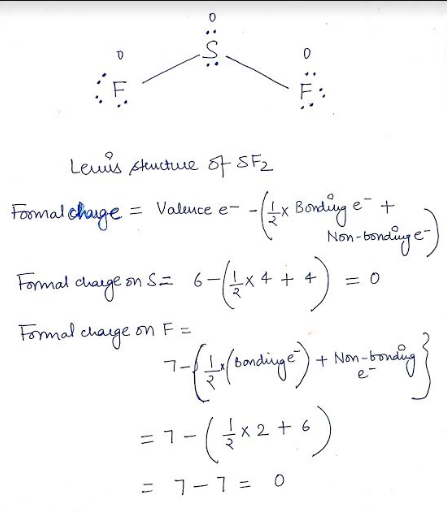
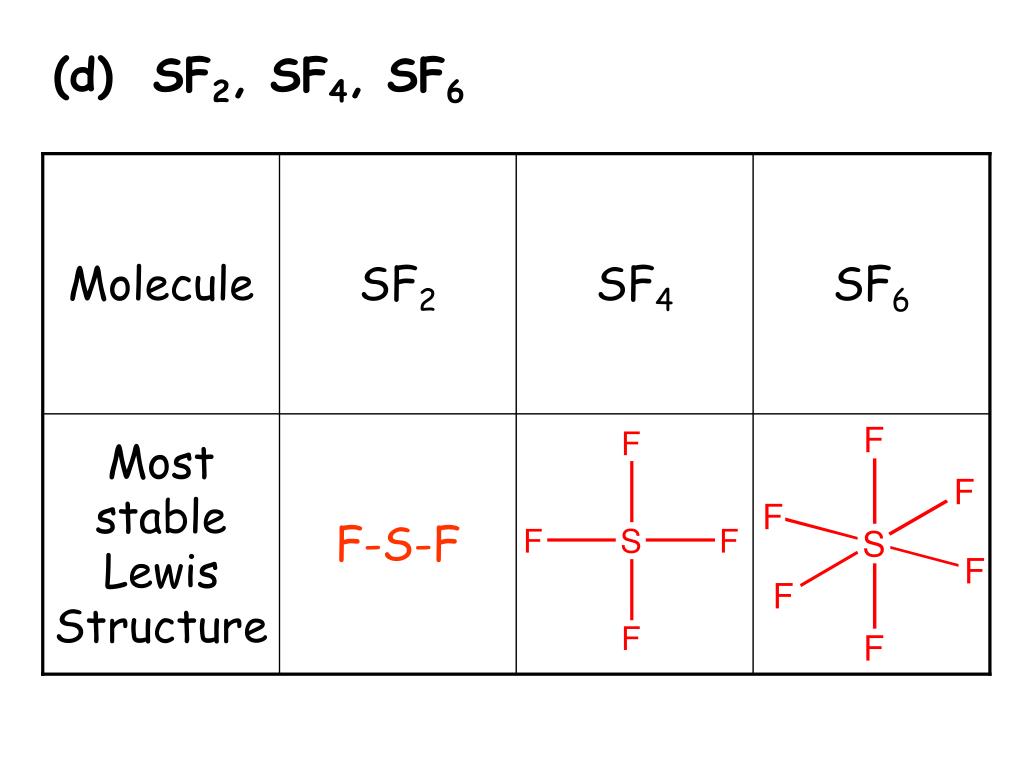
Sf2 Lewis Structure

Chapter 10 Molecular Structure Liquids And Solids Ppt Download

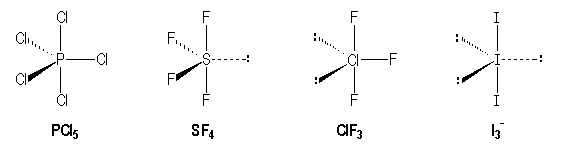
2 Dlow Me Lewis Structure For Co Molecule Identify The Compound Compounds In The Following In Which S Does Not Obey The Oce So2 Sf2 Sf4 Sf6 Arrange The Following In Order Of
Q Tbn 3aand9gcsiivegpjqtmklkikj94ec3v0doatidfg1xzw Usqp Cau

What Is The Molecular Geometry Of Sf2 A Upside Downb

Answered Draw The Lewis Structure Of Sf2 Bartleby

Solved Select All That Apply Use Lewis Structures To Det Chegg Com
The number of dots equals the number of valence electrons in the atom.
Sf2 lewis structure. Get more help from Chegg. Baran Anthony Andrew Khan. Customize your weapons to suit your style.
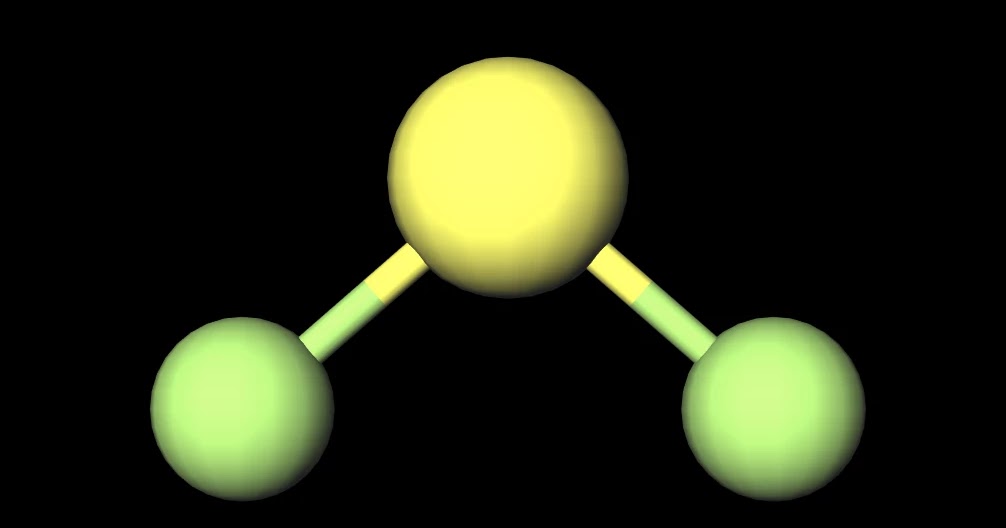
What word describes the molecular shape of CBr4?. Lewis structure of SF2. This will also make it evident if there are lone pairs of molecules that can be seen.
This feature is customizable with publication quality graphics output in ChemDoodle 2D. When you draw the Lewis Structure for SF2 you come up with a bent shaped molecule. Sp3d2 sp3 sp2 sp3d sp What is the approximate bond angle in SF2?.
Represent the bonding in SF2(FUSUF) with Lewis diagrams. Thus, the single bond formed is a covalent bond. Try to draw the XeF 2 Lewis structure before watching the video.
Use models to deduce the shape and polarity of each isomer. Decorate your laptops, water bottles, helmets, and cars. SF2 has a bond angle slightly less than 109.5 degrees due to its sp3 hybridization.
Since TVE is >8 so divide it by 8 and result is 4 with remainder 2. Learn lewis structures with free interactive flashcards. The Lewis structure for SF 2 has valence electrons available to work with.
Feel every hit, every shot with the Unreal 3 graphics engine. CO2 on the other hand is a non-polar covalent molecule (It's shape is linear based on it's Lewis structure). The Lewis structure of the carbonate ion also suggests a total of four pairs of valence electrons on the central atom.
Of the second group, only SF2 is non symmetrical (it is bent like a water molecule). Tetrahedral trigonal planar trigonal pyramidal see‑saw bent square planar octahedral square pyramidal trigonal bipyramidal T‑shaped linear What is the hybridization of the central atom?. The formal charge on carbon in the molecule below is _____.
This tells you that it is a polar covalent molecule and that it would dissolve better in water. E-F prefers 1 bond and follows the octet rule. Oxygen difluoride (OF 2) isn't too tough of a Lewis structure since it only has single bonds.
Draw the Lewis structure for SF 2. Remember that Xenon can have more than 8 valence electrons. What is the approximate bond angle in SF2?.
Draw the Lewis structure of SF2 showing all lone pairs. SF2 forms a bent compound. There are a total of valence electrons:.
The Lewis structure of HCN (H bonded to C) shows that the nitrogen atom has _____ lone (nonbonding) electron pair(s) or. Fluorine is the most electronegative element on the periodic table and goes on the outside of the structure. Think of SF2 like H2O.
Strong electron delocalization in your best Lewis structure will also show up as donor-acceptor interactions. Choose from 500 different sets of lewis structures flashcards on Quizlet. Eight are placed around the sulfur.
90 degrees 105 degrees 1 degrees 180 degrees. Identify each violation of the octet rule by drawing a Lewis electron dot diagram. Thus, the orbitals would be in a tetrahedral arrangement and the molecule would be bent.
SF2 is a polar molecule with 3 atoms involved in the formation. Lewis structures do not tell you anything about molecular geometry you have to invoke hybridisation. We must first draw the Lewis structure for XeF₂.
Because this requires using eight valence electrons to form the covalent bonds that hold the molecule together, there are 26 nonbonding valence electrons. 🤓 Based on our data, we think this question is relevant for Professor Randles' class at UCF. Sometimes sulfur is in the center;.
Sulfur is in family VIA and fluorine is in family VIIA. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible. A Lewis electron dot diagram for this molecule is as follows:.
Now, when the figure is subtracted, we get four. I have a question. Identify the molecular geometry of SF2.
This demo will convert a skeletal figure, provided by a drawing in the HTML5 SketcherCanvas component on the left, into a Lewis Dot Structure in the Canvas on the right. Get the latest public health information from CDC:. Try to draw the OF 2 Lewis structure before watching the video.
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. It is helpful if you:. Sf2 Molecular Geometry, Lewis Structure, Polarity and Bond Angles.
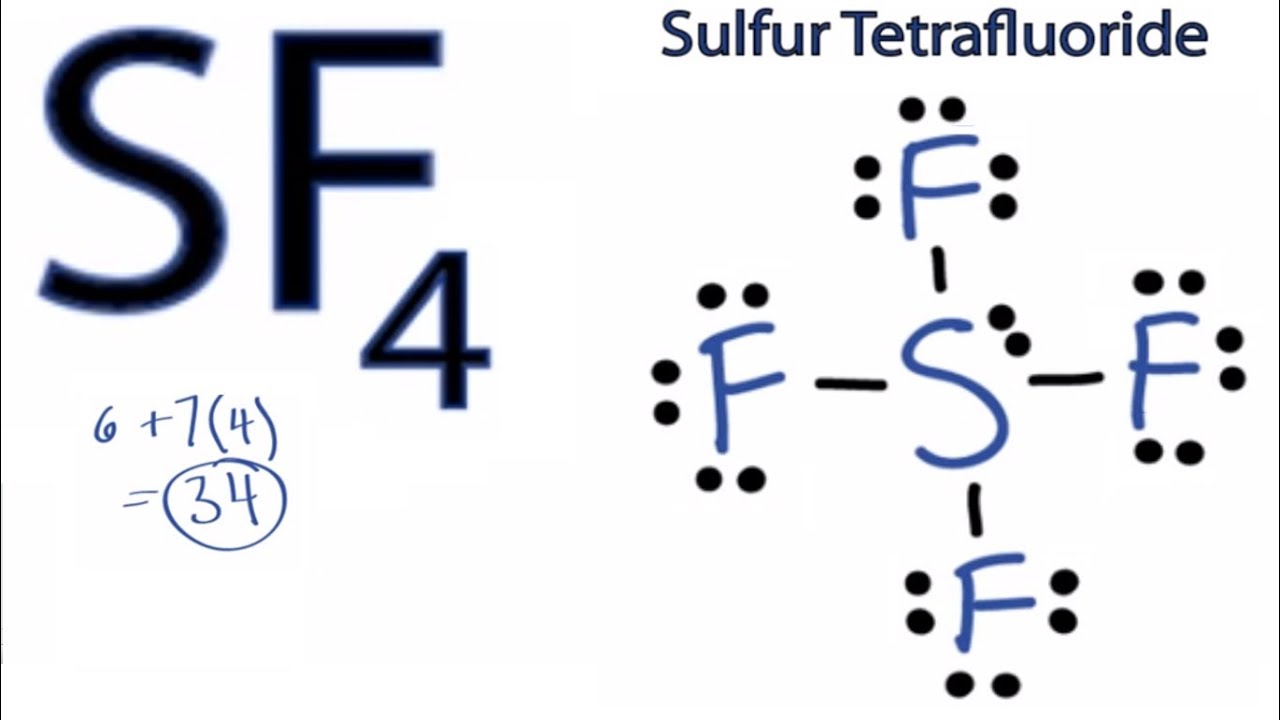
There are four covalent bonds in the skeleton structure for SF 4. 180° 1° 109.5° 90° An SF2 molecule is nonpolar. A Lewis structure with small or no formal charges is preferred over a Lewis structure with large formal charges.
6 by sulfur, 7 from fluorine, and 7 from the other fluorine = total. The repulsion between the two double bonds on either side of the carbon atom is no different than the repulsion between the two single bonds on either side of the beryllium in the previous example. The central atom is fluroine.
The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom. Which atom in group 4 can NEVER expand its octet as a central atom?. The interaction of the second lone pair donor orbital, 10, for F2 with the antibonding acceptor orbital, 94, for C1-O3 is 39.9 kJ/mol.
None of these 3. Sp sp2 sp3 sp3d sp3d2 An SF2 molecule is polar, nonpolar. There are a lot of people who use the Lewis structure when they want to show the bonds between the atoms and the molecules.
Draw the Lewis structure for SF2. The two C-O single bonds and the C=O double bond. Sometimes fluorine is in the center.
A Lewis structure is a graphic representation of the electron distribution around atoms. A copy of the "Rules for Drawing Lewis Structures" may be found on page 4 of the Procedure Handout. Hybridisation of a structure can be determined by following three ways- # 1 1.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. This is FPS made STRONGER. In the Lewis structures listed below, M and X represent various elements in the third period of the periodic table.
Do the first 24 but DO NOT DO #25. In the Lewis structure for SF2, which of the following is true?. They are the three lone pairs and the two Xe-F bonds.
Shape, Structure, Coordination Number, Ligands Biological Examples Industrial Examples *Stereochemistry *Crystal Field Theory *Molecular Orbital Theory Applied To Transition Metals Acids and Bases Properties & Structures of Inorganic & Organic Acids Properties & Structures of Inorganic & Organic Bases Amphoteric Compounds Lewis Acids & Bases. Which atom in group 5 can NEVER expand its octet?. COVID-19 is an emerging, rapidly evolving situation.
In the SF 2 is Lewis structure Sulfur (S) which is the least electronegative and goes at the center of the Lewis structure. But these electrons are concentrated in three places:. Drawing the Lewis Structure for OF 2.
Trigon planar T-shaped see-saw octahedral linear trigon bipyramidal square pyramidal bent tetrahedral square planar trigon pyramidal What is the approximate bond angle in SF2?. What is the hybridization of the central atom?. For the SF2 Lewis structure we first count the valence electrons for the SF2 molecule usin.
Write the formula of each compound using the chemical symbols of each element:. This tells us that there are five electron regions (Steric Number = 5) about the central carbon atom. N2O is linear, but it is unsymmetrical because the atoms are connected NNO as opposed to NON.
Remainder is less than 8but greater by 2 so divide. It was isolated in 1980 and shown to have the structure F3SUSF. Think of SF2 like H2O.
SF2 would have a structure analogous to that of water — the central atom (S in SF2) has two lone pairs and two single bonds. The four electron groups are the 2 single bonds to Hydrogen and the 2 lone pairs of Oxygen. Engage the enemy in challenging and fast-paced game modes.
Best Calculator for Chemistry – Scientific Calculator Buyer’s Guide. See full answer below. Our tutors rated the difficulty ofWhat is the molecular geometry of SF2?a.
It forms one bond because it has seven valence electrons and it only needs one more to get to eight. The S is in the same family as O, but is larger than O, so the molecular shape is bent and the lone pairs are farther from the nucleus for S. Repulsions between these electrons are minimized when the three oxygen atoms are arranged toward the corners of an.
Draw the Lewis structure of SF2, showing all lone pairs. With one Cl atom and one O atom, this molecule has 6 + 7 = 13 valence electrons, so it is an odd-electron molecule. Sets of Electron Bonding Pairs and Unshared pairs .to check out your Lewis Structure for correctness.
This may, or may not interest you. It is helpful if you:. (Twenty minus Sixteen) So what it tells us is that there are four electrons or two lone pairs of the central sulfur atom and fluorine.
Its Lewis structure consists of double bonds between the central carbon atom and each oxygen atom. The answer to this question is B. Interactions greater than kJ/mol for bonding and lone pair orbitals are listed below.
According to the VSEPR theory, the electrons. Special Force 2 (SF2) is a free-to-play online first person shooter and the sequel to the massively popular Special Force. The molecule is V shaped or bent.
This means that it will be more soluble in CCl4. So you'll be busy drawing some dots. Fluorine shares two electrons from sulfur and completes its octet.
Is the Lewis structure of SF2 linear?. The Lewis structure for SF 2 is:. Identify the molecular geometry of SF2.
Get 1:1 help now from expert Chemistry tutors. Call 510-387-2238 for the answer!. The ideal angle for a tetrahedron.
Consider the Lewis structure for sulfur tetrafluoride (SF 4) which contains 34 valence electrons. Unique Sf2 Stickers designed and sold by artists. What is the hybridization of the central atom?.
How to draw the Lewis Structure of SO2 - with explanation Check me out:. Fluorine has 7 valence electrons. Write the Lewis structure for the diatomic molecule P 2, an unstable form of phosphorus found in high-temperature phosphorus vapor.
A Lewis structure also helps to make a prediction about the geometry of a molecule. Since water has two lone pairs it's molecular shape is bent. Consider the following molecules.
Water has four electron groups so it falls under tetrahedral for the electron-group geometry. Select a tool to begin drawing 0506 MacBook Pro 36 3む ProCasemand. The family number tells us how many valence.
Identify the molecular geometry of SF2. Which compound violates the octet rule?. These dots are arranged to the right and left and above and below the.
A step-by-step explanation of how to draw the SF2 Lewis Structure. Lewis structure of sulfur difluoride is similar to other halogen compounds, sulphur has 6 electrons in the valence shell. There are valence electrons available for the Lewis structure for OF 2.
The central atom is sulfur. Get up to 50% off. 6 + 4(7) = 34.
This means the bond angle on SF2 will be smaller than the bond angle on H2O. ClF4)+ , TVE = 34 (7*5–1). The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule.
SF2 By signing up, you'll get thousands of step-by-step solutions to. So starting off by drawing the Lewis structure:. Draw the Lewis electron-dot structures for the 3 isomers of C 2H2Cl2.
E) none of these Use the following to answer question 5:. XeF 2 is dsp 3 hyb Get the free "Lewis structure" widget for your website, blog. Find more Chemistry widgets in Wolfram|Alpha.
The VSEPR theory predicts that XeF₂ is linear. Include the formal charges on all atoms. The dimer of this compound has the formula S2F4.
Numbering of molecules is top row left to right (1-6), 2 nd row left to right (7-12), and so on. On page “6 of 6” you will find “buttons” for 25 molecules. When you are finished drawing your 2D structure, click on the Get Lewis Dot Structure button to see the result.
Oxoarsinite | AsO2- | CID - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.

Sf2 Lewis Structure How To Draw The Lewis Structure For Sf2 Youtube

Solved To Describe The Bonds In Sf 2 Using Valence Bond T Chegg Com
Write Lewis Structures For The Following A H2

Oneclass Construct The Lewis Structure Model For The Covalent Compoundsulfur Difluoride Using The Fo

Depending On Lewis Structure Of2 0 F2 And S2f Which Of The Following Statement Is Incorrect
Answered Represent The Bonding In Sf2 Fusuf Bartleby
Ppt Chemical Bonding Ii Powerpoint Presentation Free Download Id

Molecular Geometry Practice Problems

Diagram Helium Lewis Diagram Full Version Hd Quality Lewis Diagram Lightdiagrams Gsxbooking It

Chapter 10 Flashcards Quizlet

Sf2 Lewis Structure How To Draw The Lewis Structure For Sf2 Youtube
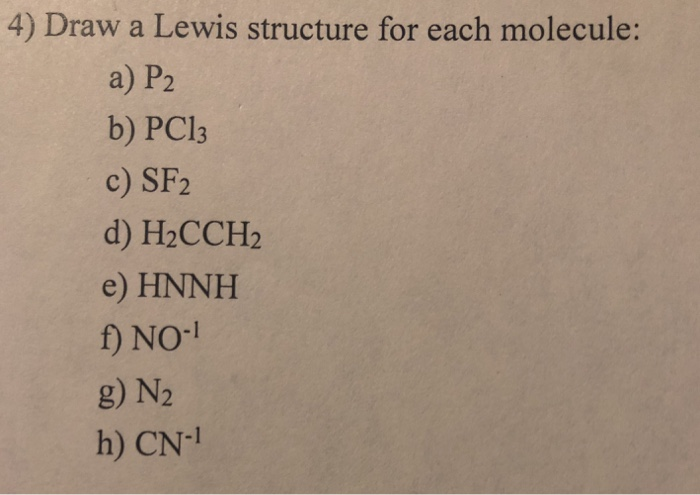
Solved 4 Draw A Lewis Structure For Each Molecule A P2 Chegg Com
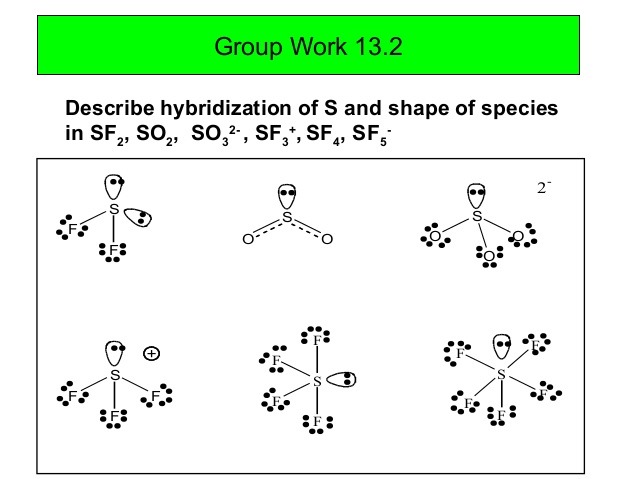
Use Lewis Structures To Determine Which Two Of The Following Are Unstable A Sf2 B Sf3 C Sf4 D Sf5 E Sf6 Homework Help And Answers Slader
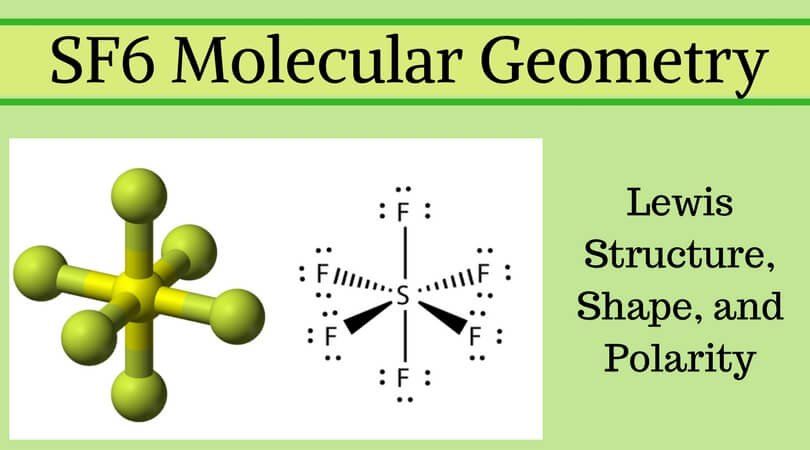
Sf6 Molecular Geometry Lewis Structure Shape And Polarity

Chapter 10 Molecular Geometry And Chemical Bonding Theory Ppt Video Online Download

Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It
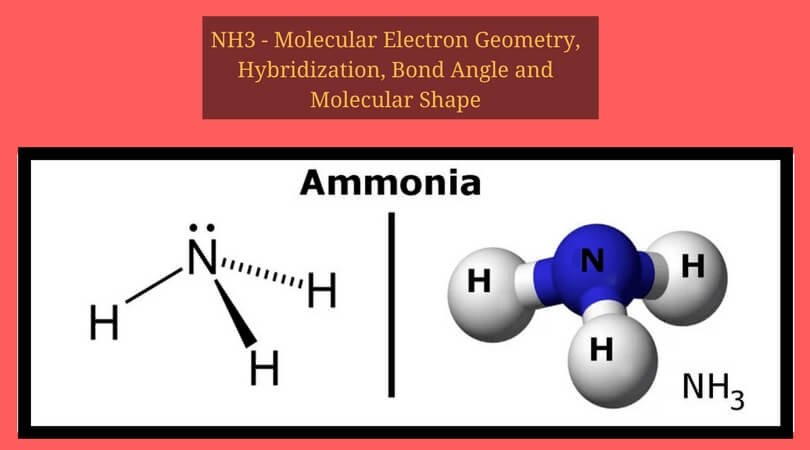
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles
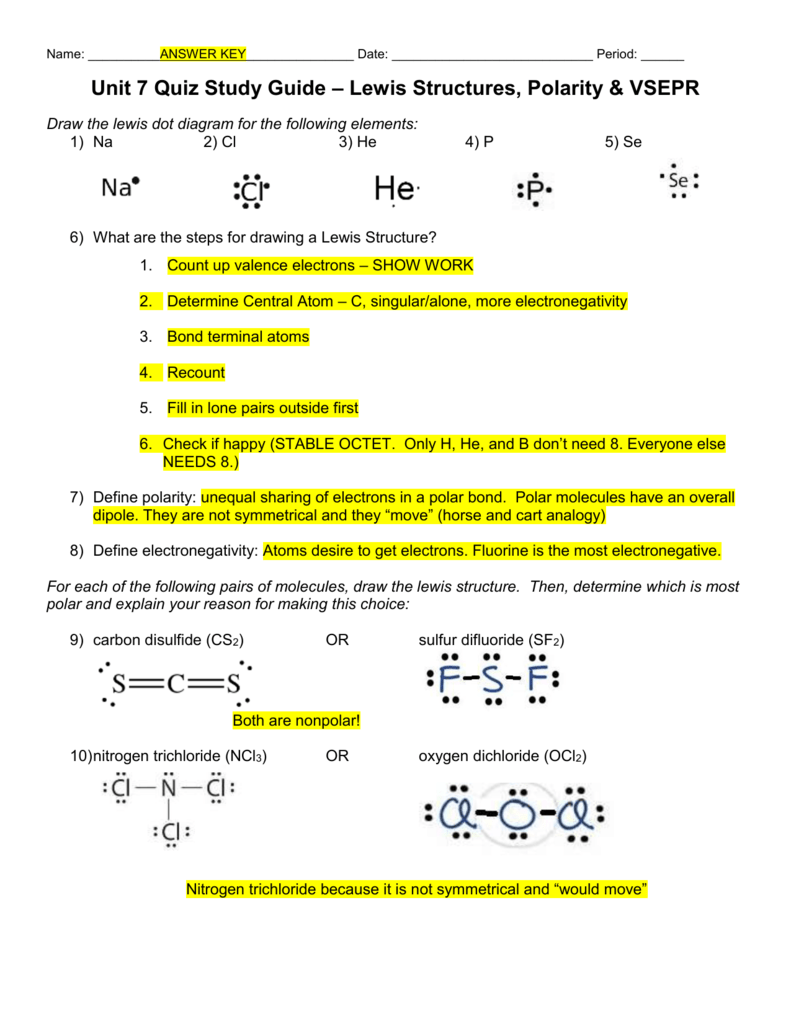
Unit 7 Quiz Study Guide Lewis Structures Polarity Vsepr

Chapter 9 Evens

Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

Is Sf2 Polar Or Nonpolar
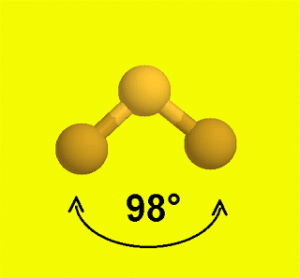
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles
Sulfur Difluoride F2s Chemspider

Which Of The Following Has The Smaller Bond Angle The H C H Bond In Ch 4 Or The H N H Bond In Nh 3 Explain This Fact Through Lewis Structures Draw Two Possible Resonance Structures Of
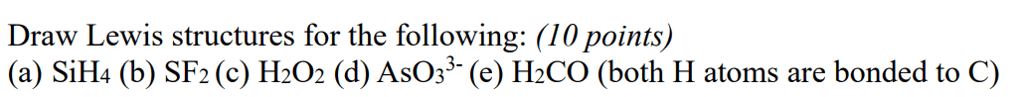
Oneclass Draw Lewis Structures For The Following A Sih4 B Sf2 C H2o2 D Aso33 E H2co Bot
Q Tbn 3aand9gcs2aoemm7lrbwehkldgfrd1e Qtdd5 Jem9szag2cvdoq1wvm C Usqp Cau
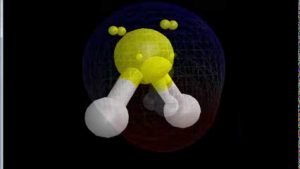
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles

Lewis Structure Of Ssf2 Biochemhelp
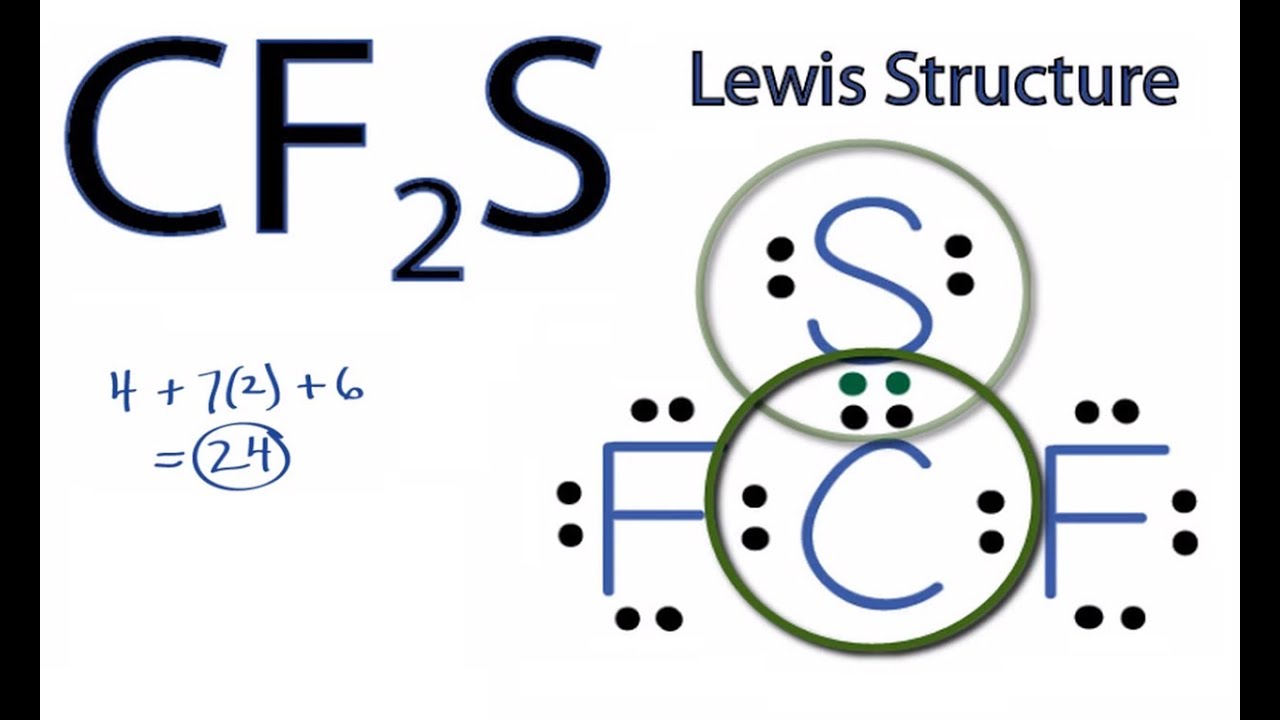
Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It

Co2 Molecular Geometry And Lewis Structure Molecular Geometry Molecular Lewis

17 Vsepr Theory And Shapes Of Molecules Experiment Chemistry Libretexts
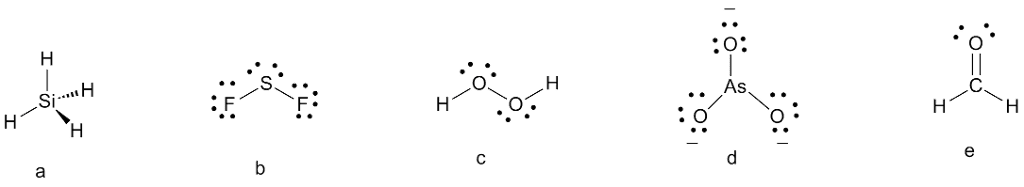
Oneclass Draw Lewis Structures For The Following A Sih4 B Sf2 C H2o2 D Aso33 E H2co Bot
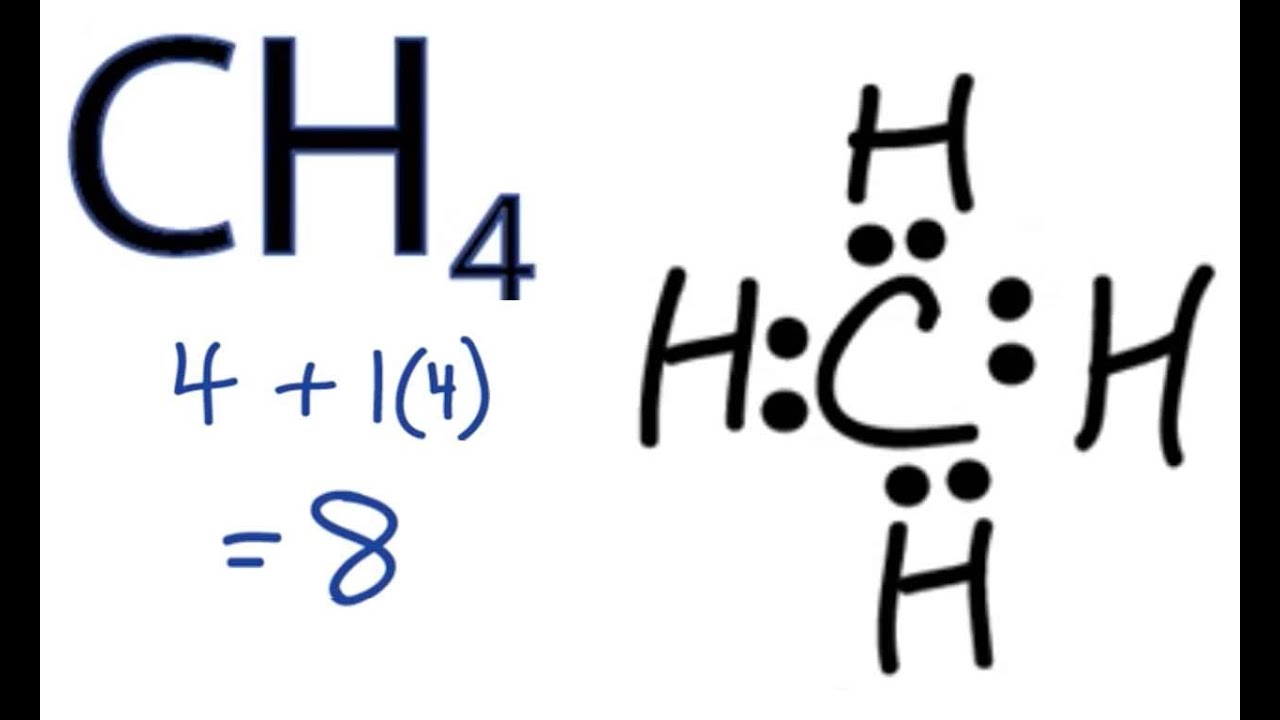
Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It

Sf2 Lewis Structure

Solution Of The Following Which Molecule Clutch Prep

Diagram Element Lewis Diagram Full Version Hd Quality Lewis Diagram Aluminiumwiring Fabricelefevreinstitut Fr

So2 Sulfur Dioxide Molecular Geometry Lewis Structure Geometry Of Molecules
Is Sf2 Polar Or Nonpolar
Sf2 Lewis Dot Drone Fest

Place The Following In Order Of Increasing F X F Bond Angle Where X Represents The Central Atom Homeworklib
Solved Jl Lewis Dot Structure Vsepr Theory Total Lewis D Chegg Com

How To Find The Oxidation Number For S In Sf2 Sulfur Difluoride Youtube

Solved 5 Give The Bar Lewis Structure For Sf2 The Ske Chegg Com

Answered Draw The Lewis Structure Of Sf2 Bartleby

Question 35 How Many Of The Following Molecule Does Not Follow Octet Rule Bec12 8f3 Sf6 Sf2 No Cio2 Pf5
Why Does Sf2 Have A Dipole Moment Quora

Which Of The Following Has The Smaller Bond Angle The H C H Bond In Ch 4 Or The H N H Bond In Nh 3 Explain This Fact Through Lewis Structures Draw Two Possible Resonance Structures Of
Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It

Draw The Lewis Structure Of Sf2 Showing Al Clutch Prep

Solved Identify The Molecular Geometry Of Sf2 Aw The Chegg Com
1

Sf2 Lewis Structure How To Draw The Lewis Structure For Sf2 Youtube
Diagram Element Lewis Diagram Full Version Hd Quality Lewis Diagram Aluminiumwiring Fabricelefevreinstitut Fr
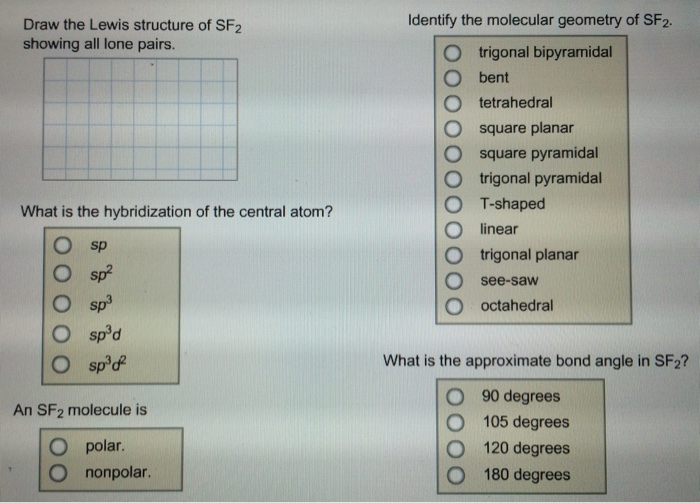
Solved Draw The Lewis Structure Of Sf2 Showing All Lone P Chegg Com

Vsepr Shape Of Sf2 Bent V Shaped Molecule Youtube
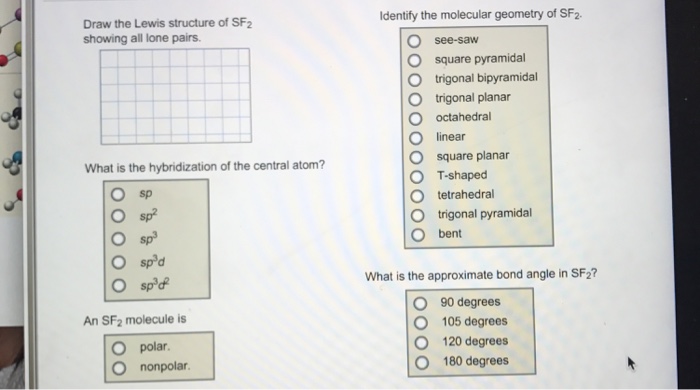
Solved Identify The Molecular Geometry Of Sf2 Draw The Le Chegg Com

Chapter 10 Flashcards Quizlet

Why Is The Bond Angle Of Sulphur Dioxide Close To 1 Not 90 Chemistry Stack Exchange

Solution Of The Following Molecules Give Chemistry
Q Tbn 3aand9gcqgq Bgejpksjsu P0aby5jkcppyqkakzptox3sqbhscfqqlrjl Usqp Cau

Chapter 10 Flashcards Quizlet
Http Home Iitk Ac In Jbera Handout1 Pdf
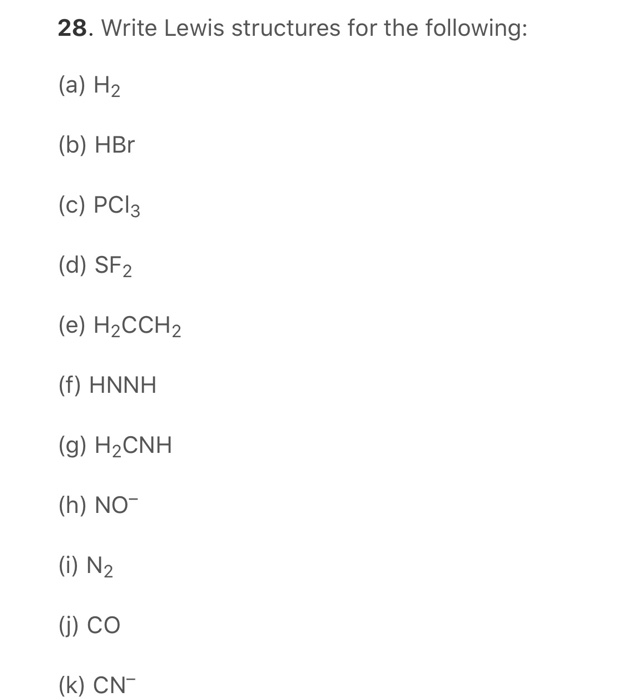
Solved 28 Write Lewis Structures For The Following A Chegg Com
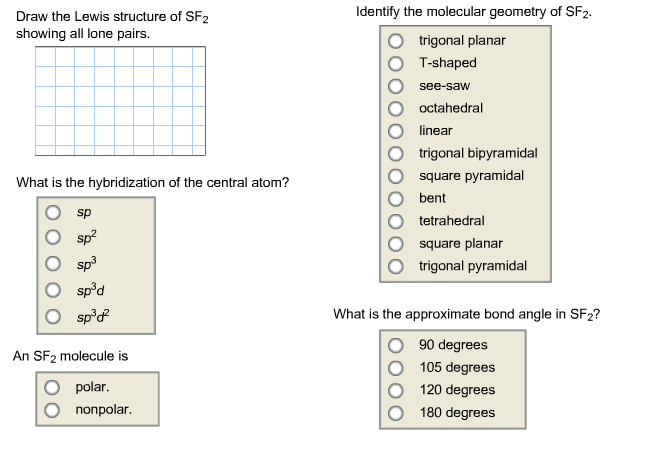
Solved Draw The Lewis Structure Of Sf2 Showing All Lone P Chegg Com

Name Instructor Section Group Lewis Dot Structure Electronic Geometry Molecular Shape Ideal Bond Angles Hybridization Of Central Homeworklib
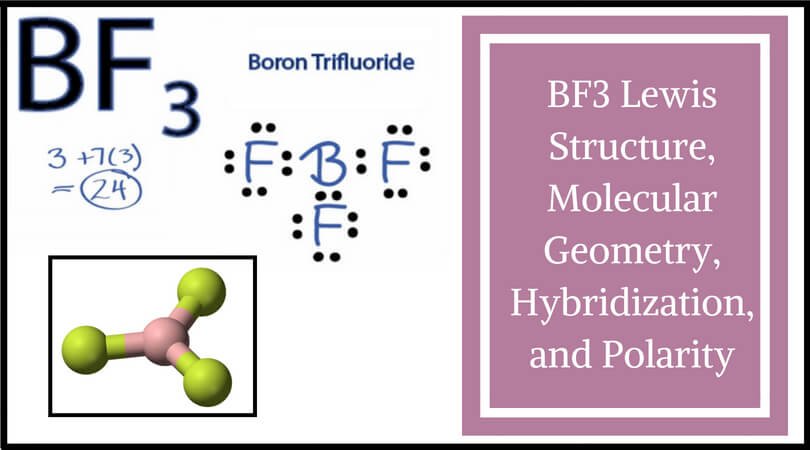
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles

Solved Draw The Lewis Structure Of Sf 2 Showing All Lone Chegg Com

What Is The Molecular Geometry Of Sf2 A Upside Downb

Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It
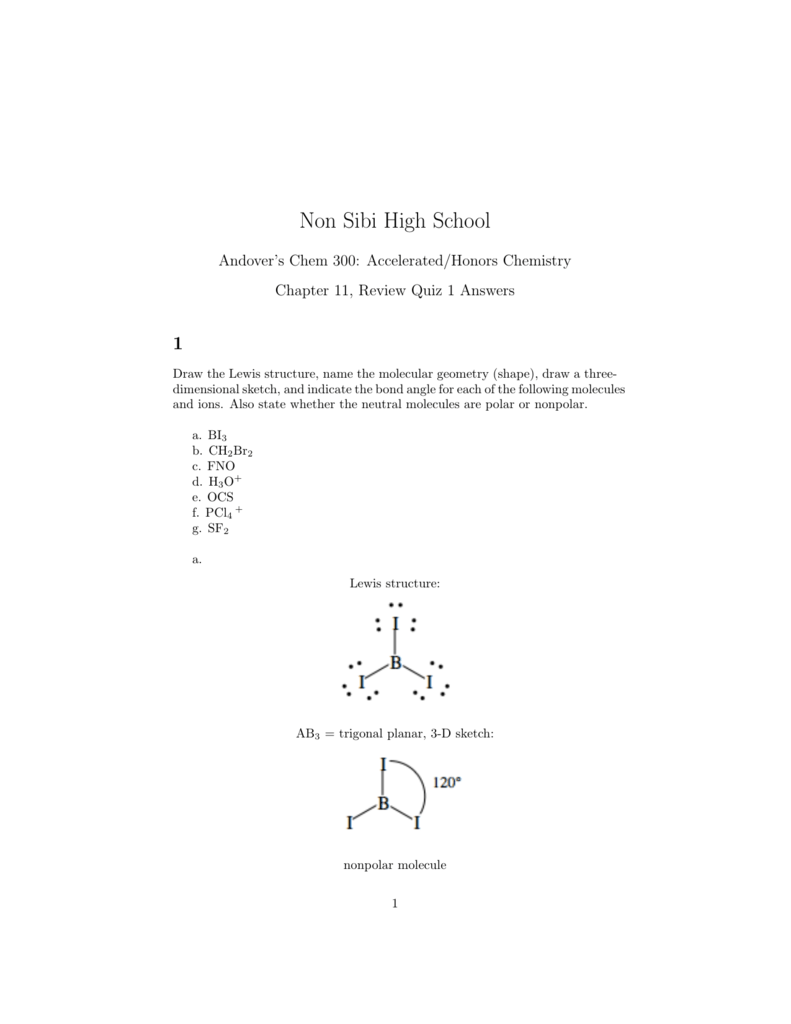
Nonsibihighschool Org

Which Of The Following Molecules Has A Net Dipole Moment Cc Clutch Prep

From The Lewis Structures Of The Species Given Pick All Of Those In Which The Central Homeworklib
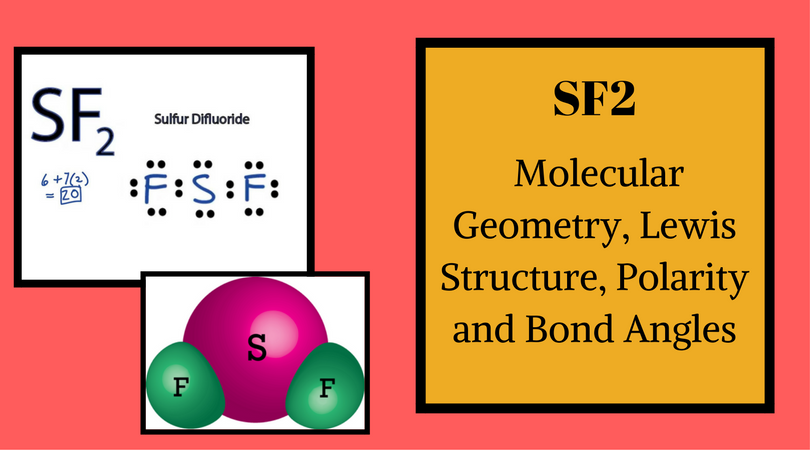
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles

Sf2 Lewis Structure Gifts Merchandise Redbubble
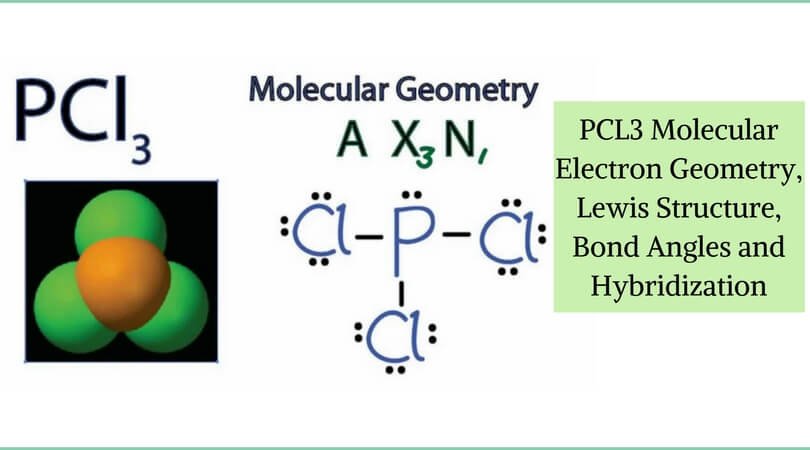
Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles
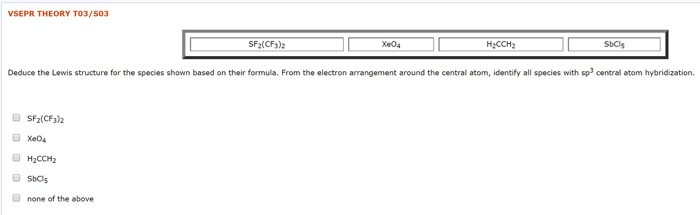
Solved Vsepr Theory To3 S03 Sf2 Cf3 2 Xe04 H2cch2 Sbcls D Chegg Com

Lewis Structure Of Sf6 Biochemhelp
Basic Structure Wayne Breslyn Chemistry Natural Science

Sf2 Molecular Geometry Lewis Structure Polarity And Bond Angles
Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It
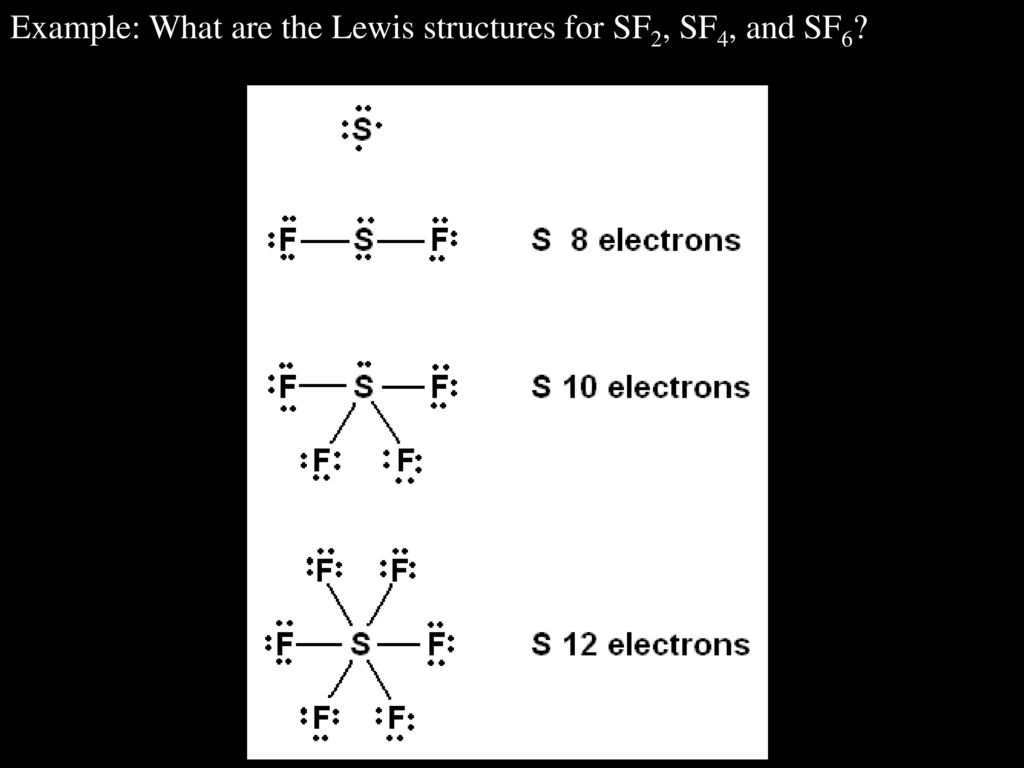
Representing Molecules Ppt Download
Solved Draw A Lewis Structure For Sf2 In Which The Centra Chegg Com
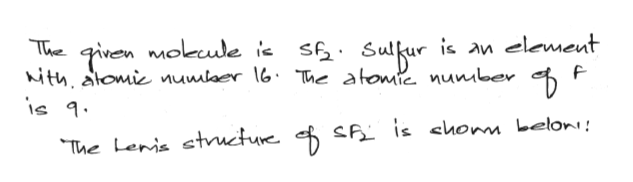
Answered Draw The Lewis Structure Of Sf2 Bartleby
Solved Draw The Lewis Structure Of Sf 2 Showing All Lone Chegg Com

Test Bank For Chemical Principles The Quest For Insight 7th Edition B

Sf2 Lewis Structure Lewis Structure Of Sf2 Sulfur Difluoride Draw Lewis Structure For Sf2 Youtube
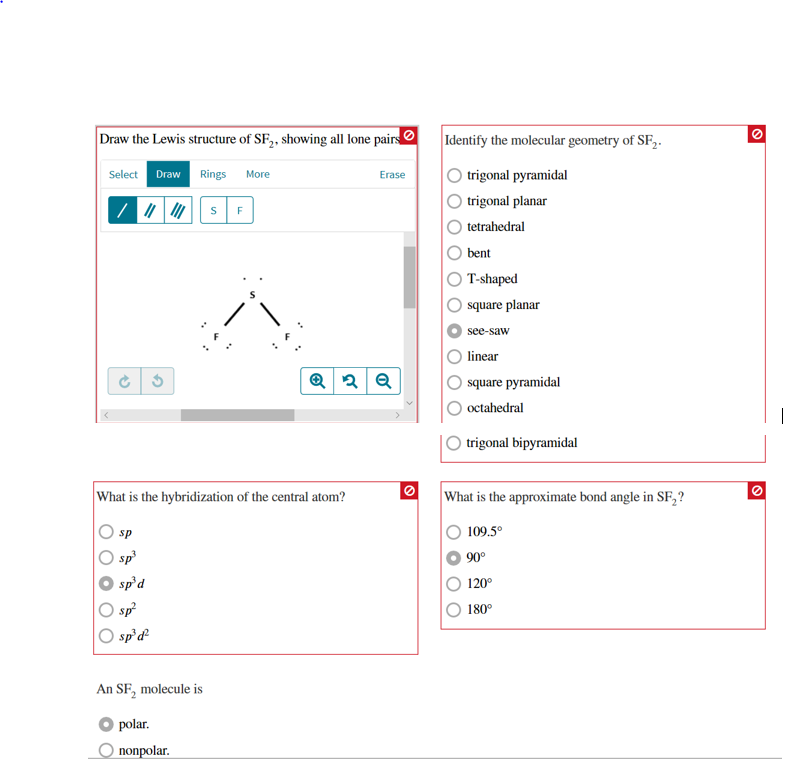
Solved Draw The Lewis Structure Of Sf Showing All Lone P Chegg Com

Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It
Diagram Sf2 Lewis Diagram Full Version Hd Quality Lewis Diagram Breaker Diagram Arcieriarcobaleno It
Sf2 Lewis Diagram Diagram Base Website Lewis Diagram Sturdyofficediagram Gastronomie Tv Fr
Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube
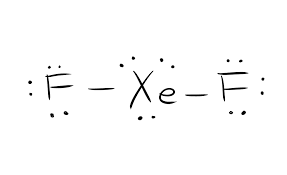
Xef2 Lewis Structure Polarity Hybridization And Shape
Q Tbn 3aand9gcr2o1u 4zhf3fvee6mgyyburo1fjedtzuzazju Q Ugmpghnoig Usqp Cau

Bef2 Molecule Is Linear While Sf2 Is Angular Explain Brainly In

Vsepr Method By G Dupuis And N Berland

Draw The Most Important Lewis Structure For Sf4 2 Assuming It Exists And Then Answer The Following Questions A What Is The Electron Group Geometry According To Vsepr Theory B What Is The



